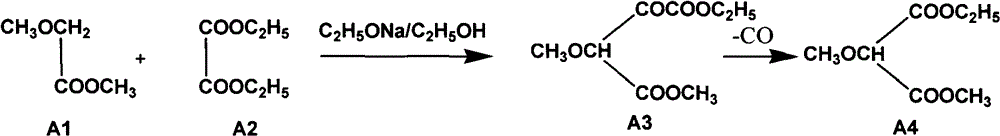Preparation method of sulfadoxine
A kind of technology of cycle-acting sulfonamide and aminobenzene sulfonamide group, which is applied in the field of preparation of sulfonamide drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 prepares 2-methoxy-methyl ethyl malonate (A4)
[0049] (1) The reaction equation is as follows:
[0050]
[0051] (2) See Table 1 for the feeding ratio.
[0052] Table 1
[0053] raw material name Feeding amount (kg) molecular weight The molar ratio of A1 (methyl methoxyacetate) 175 104 1 A2 (diethyl oxalate) 280 146 1.14 Sodium ethylate solid 130 68 1.12
[0054] (3) The specific operation process is as follows:
[0055] Dry the reaction kettle (normal batch reaction reaction kettle does not need to be washed), mix methyl methoxyacetate and diethyl oxalate in a mixed ester pot, and cool to below 10°C, add to the reaction kettle, under stirring conditions, Pour the solid sodium ethoxide into the reaction kettle (the package of sodium ethoxide should not be disassembled, and the minimum package of sodium ethoxide should be used as the unit when feeding, and poured in several times), the rea...
Embodiment 2
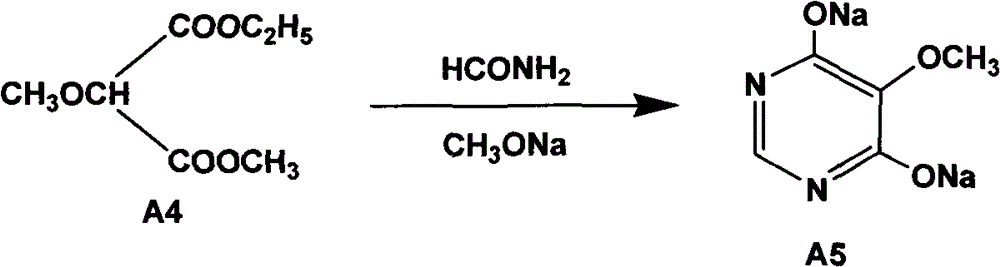
[0058] Embodiment 2 prepares cyclic compound-hydroxyl sodium salt (A5)
[0059] (1) The reaction equation is as follows:
[0060]
[0061] (2) The feeding ratio is shown in Table 2.
[0062] Table 2
[0063] raw material name Feeding amount (kg) molecular weight The molar ratio of A4 (Gradestone) 240 176 1 Formamide 186 45 3.37 liquid sodium methoxide 830 54 3.46
[0064] (3) The specific operation process is as follows:
[0065] Dry the reactor first, put in liquid sodium methoxide, stir, heat to 60-68°C, add formamide, then add A4 obtained in step (1) evenly, control the temperature when adding to 65-70°C, and add time for 1-68°C After 1.5 hours, the addition is completed, and the heat preservation reaction is more than 0.5 hours. Recover methanol at room temperature, then recover methanol under reduced pressure until it does not come out, then add 1000L of water, recover methanol with water until clarific...
Embodiment 3
[0066] Embodiment 3 prepares chloride (A6)
[0067] (1) The reaction equation is as follows:
[0068]
[0069] (2) The feeding ratio is shown in Table 3.
[0070] table 3
[0071] raw material name Feeding amount (kg) molecular weight The molar ratio of A5 (the ring compound that embodiment 2 prepares) 240 186 1 Phosphorus oxychloride 825 153 4.17 Trichlorethylene 900 131 /
[0072] (3) The specific operation process is as follows:
[0073] In the reaction pot (covered tightly), press the pre-prepared phosphorus oxychloride. Turn on the reflux and hydrogen chloride gas absorption device, heat to 40°C with steam, turn off the steam to raise the temperature naturally, start to slowly add the cyclic compound-hydroxy sodium salt (A5), and control the adding speed, (pay attention to the sudden temperature phenomenon when adding, About ten to twenty minutes), add the rest of the cyclic compound-hydroxyl sodium sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com