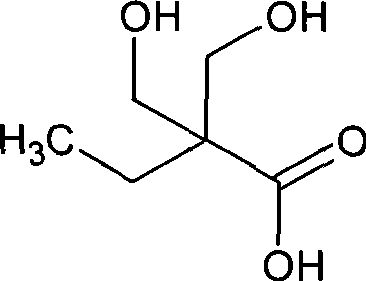
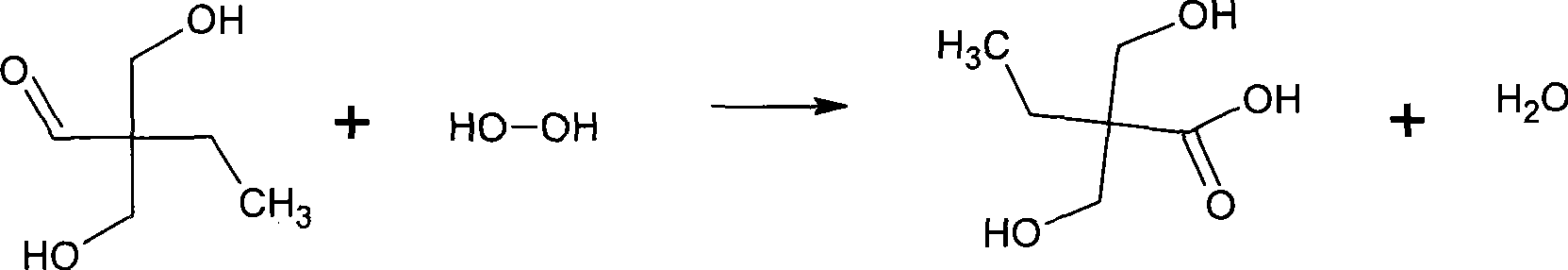
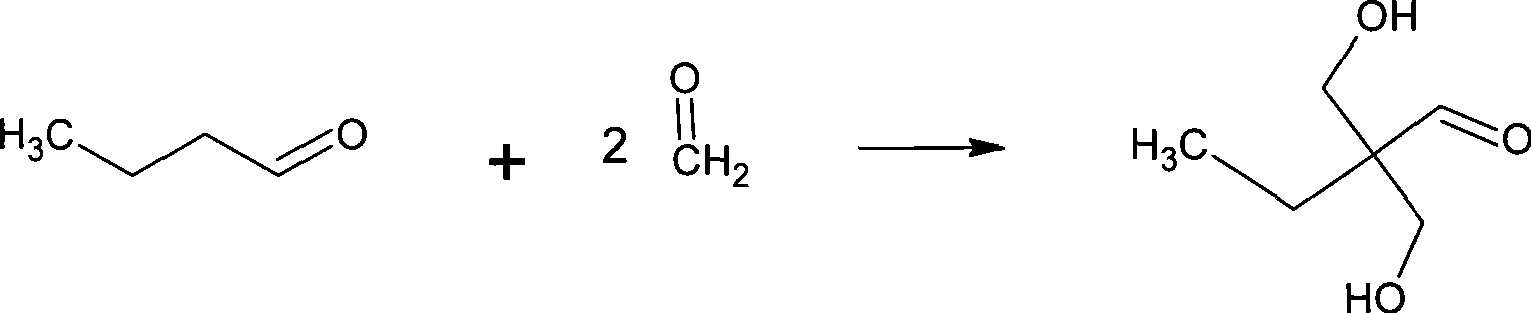Method for synthesizing 2,2-bis(hydroxymenthyl)butyric acid
A technology for the synthesis of dimethylolbutyric acid, which is applied in the fields of oxidative preparation of carboxylic acids, organic chemistry, etc., can solve the problems of reduced yield and high content of by-products, and achieve low cost, high oxidation efficiency, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Add 37% formaldehyde aqueous solution and catalyzer in the 250ml four-neck flask that agitator, reflux condenser, dropping funnel and thermometer are equipped, the ratio of formaldehyde and catalyzer is 1:0.02, molar ratio; Described catalyzer is Ba( Oh) 2 ;
[0064] Propionaldehyde is added in the dropping funnel, the ratio of formaldehyde and butyraldehyde is 2:1, molar ratio;
[0065] Start stirring, control pH=11, temperature at 25°C, slowly add butyraldehyde dropwise, keep the pH at 11 and continue the reaction for 3 hours after the dropwise addition is complete;
[0066] After the reaction, add an appropriate amount of formic acid to neutralize to pH = 7, add the reaction materials to a round bottom flask, and carry out vacuum distillation (0.4-0.8 atmospheric pressure) to remove unreacted formaldehyde and butyraldehyde; Butyl ketone was extracted three times to remove the side reaction product methacrolein. The volume ratio of extractant to mother liquor is 1:...
Embodiment 2
[0070] Add paraformaldehyde and catalyzer in the 250ml four-necked bottle that agitator, reflux condenser, dropping funnel and thermometer are equipped with, described as Na 2 CO 3 (solid); the ratio of paraformaldehyde and catalyst is 1:0.06, molar ratio;
[0071] Propionaldehyde is added in the dropping funnel, the ratio of formaldehyde and butyraldehyde is 2.1:1, molar ratio;
[0072] Start stirring, control pH = 10, temperature at 40°C, slowly add butyraldehyde dropwise, after the dropwise addition, keep the pH at 10 and continue the reaction for 4 hours;
[0073] After the reaction, add an appropriate amount of formic acid to neutralize to pH = 7, add the reaction materials to a round bottom flask, and carry out vacuum distillation (0.4-0.8 atmospheric pressure) to remove unreacted formaldehyde and butyraldehyde;
[0074] The above product is added to distilled water, and the addition amount is 3 times of the volume of the obtained solution, the volume ratio. Heat to 7...
Embodiment 3
[0078] Add formaldehyde and catalyzer in the 250ml four-neck flask that agitator, reflux condenser, dropping funnel and thermometer are equipped with, described catalyzer is triethylamine and Na 2 CO 3 (solid) mixture; the ratio of formaldehyde and catalyst is 1:0.04, molar ratio;
[0079] Propionaldehyde is added in the dropping funnel, the ratio of formaldehyde and butyraldehyde is 2.2:1, molar ratio;
[0080] Start stirring, control pH = 10, temperature at 35°C, slowly add butyraldehyde dropwise, keep the pH at 10 and continue the reaction for 5 hours;
[0081]After the reaction, add an appropriate amount of formic acid to neutralize to pH = 7, add the reaction materials to a round bottom flask, and carry out vacuum distillation (0.4-0.8 atmospheric pressure) to remove unreacted formaldehyde and butyraldehyde; Butyl ketone was extracted three times to remove the side reaction product methacrolein. The volume ratio of extractant to mother liquor is 1:1, and the extraction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com