Method for producing rotavirus vaccines in large scale by utilizing bioreactor
A bioreactor and rotavirus technology, applied in biochemical equipment and methods, viruses, microorganisms, etc., can solve the problems of low virus virulence titer, low rotavirus adsorption infection, etc., to improve yield and quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of rotavirus liquid
[0037] 1) Recovery and passage of Vero cells: After the cryopreservation tube containing the working seeds of Vero cells (the cells are from ATCC, the working seeds of the cells are self-made) is taken out from the liquid nitrogen, it is quickly put into a 40°C water bath to thaw, and then placed in the Take out the cell suspension in the ultra-clean bench, add it to the cell culture bottle, add the cell growth solution, and put it into the CO2 incubator for cultivation; after the cells grow into a single layer, digest the adherent cells and make a uniform cell suspension solution, according to the seeding ratio of 1:4, inoculated in the cell bottle, added the cell production solution, put it into the CO2 incubator to continue culturing, after two passages, inoculated the cells in the 10-layer cell factory, and After adding cell growth medium, culture;
[0038] 2) Cell collection and inoculation bioreactor: When the c...
Embodiment 2
[0045] 75L bioreactor cultivates the method for rotavirus vaccine, and preparation method is shown in embodiment 1, and wherein step 4) described bioreactor enters liquid, and the perfusion velocity of changing liquid also has effect to bioreactor production rotavirus Certain effects are shown in Table 1.
[0046] Table 1:
[0047] serial number
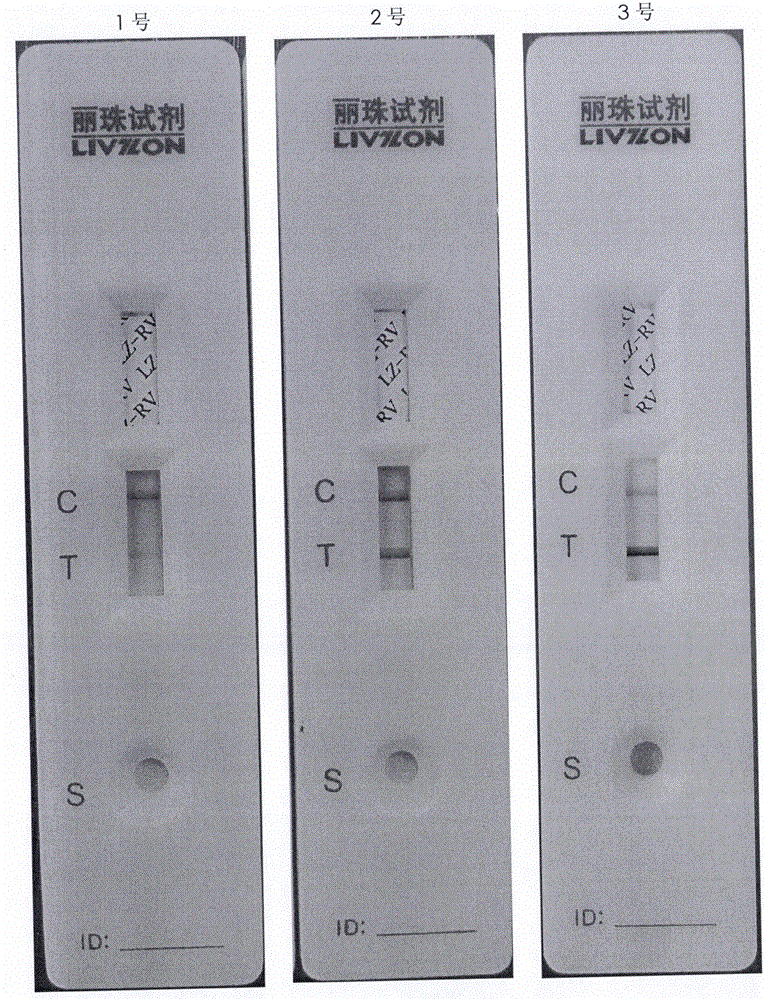
[0048] Conclusion: As shown in Table 1, compared with Group 1 and Group 5, Group 5 is only the cell growth solution in which the serum concentration in the culture solution has been reduced to 0.2% as the cell maintenance solution after infection, but the result detected by the colloidal gold kit is , 10 days after infection, the color of the sampled detection band was far lighter than that of the positive band, and the virus titer and antigen content were also very low. Therefore, whether to replace the serum-free maintenance solution before infection, and the thoroughness of the maintenance solution replacement, will dir...
Embodiment 3
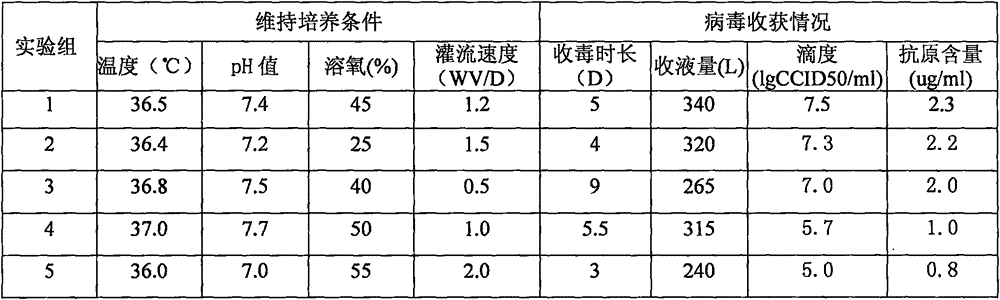
[0050] 75L bioreactor cultivates the method for rotavirus vaccine, and preparation method is the same as shown in embodiment 1, wherein step 5) described virus seed is activated and bioreactor controls the condition of the bioreactor when cell infection 24 hours after liquid change As shown in table 2
[0051] Table 2:
[0052]
[0053] Conclusion: As shown in Table 2, the virus titer of the rotavirus pooling solution harvested by groups 1-3 reaches 7.0lgCCID 50 / ml or more, the antigen content reaches more than 2.0ug / ml, which meets the requirements of large-scale production, wherein the titer and antigen content of the rotavirus pooled liquid of group 1 (i.e. embodiment 1) are optimal. And group 4, the harvest liquid that group 5 harvests, the virus titer of harvest liquid is lower, and antigen content is also lower, therefore, the control condition of the bioreactor described in step 5) is: temperature 35.0~36.0 ℃, pH The value is 7.2-7.5, the dissolved oxygen is 15%-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com