Chimeric antigen receptor of anti-human CD19 antigen and application thereof
A technology of chimeric antigen receptors and antigens, which is applied in the field of genetic engineering, can solve problems such as inability to mediate, achieve a wide range of selection, enhance the ability to proliferate and kill tumors, and have high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Screening of humanized monoclonal antibodies against human CD19 antigen
[0063] The humanized sequence of the murine anti-CD19 antibody FMC635 was replaced by screening the humanized sequence (Part 1) of the declared patent 201710301492.1, and the humanized IgG antibody molecule was designed by random mutation, as shown in Table 1. Cloned antibody random mutation results.
[0064] Table 1
[0065]
Embodiment 2
[0066] Example 2, Purification of Humanized Monoclonal Antibody Targeting Human CD19 Antigen
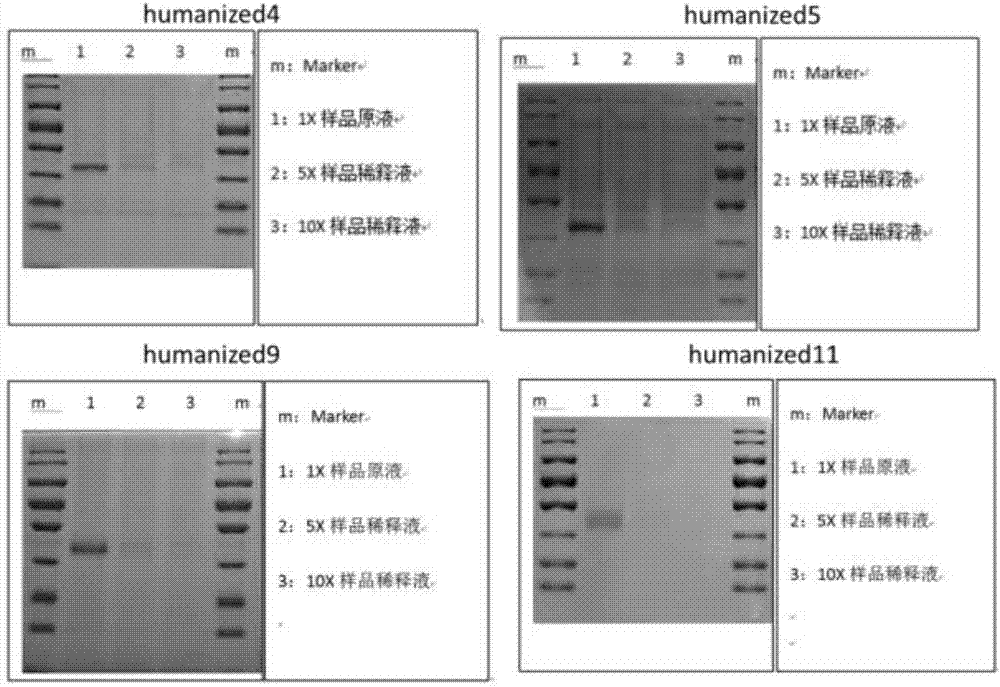
[0067] Construct a stably transfected cell line that stably expresses the humanized antibody, pass the harvested cell supernatant through a 0.45um filter, and take the filtrate. Dilute with an equal volume of equilibration buffer and measure the pH. See the GE product manual for the specific steps of purification with AKTA Prime, concentrate by ultrafiltration, sterilize and filter with a 0.22 μm syringe filter after completion, subpackage, label and store in a -80°C refrigerator. Sampling, testing. Use SDS and Western blot detection, see GE Antibody Purification Handbook for specific steps. Test results such as figure 1 As shown, four monoclonal antibodies are preferred to obtain antibodies with higher purity, which are ScFv-humanized4 amino acid sequence SEQ ID NO.1, ScFv-humanized5 amino acid sequence SEQ ID NO.2, ScFv-humanized9 amino acid sequence SEQ ID NO.3, ScFv - humaniz...
Embodiment 3
[0068] Example 3. Specific detection of humanized monoclonal antibody targeting human CD19 antigen
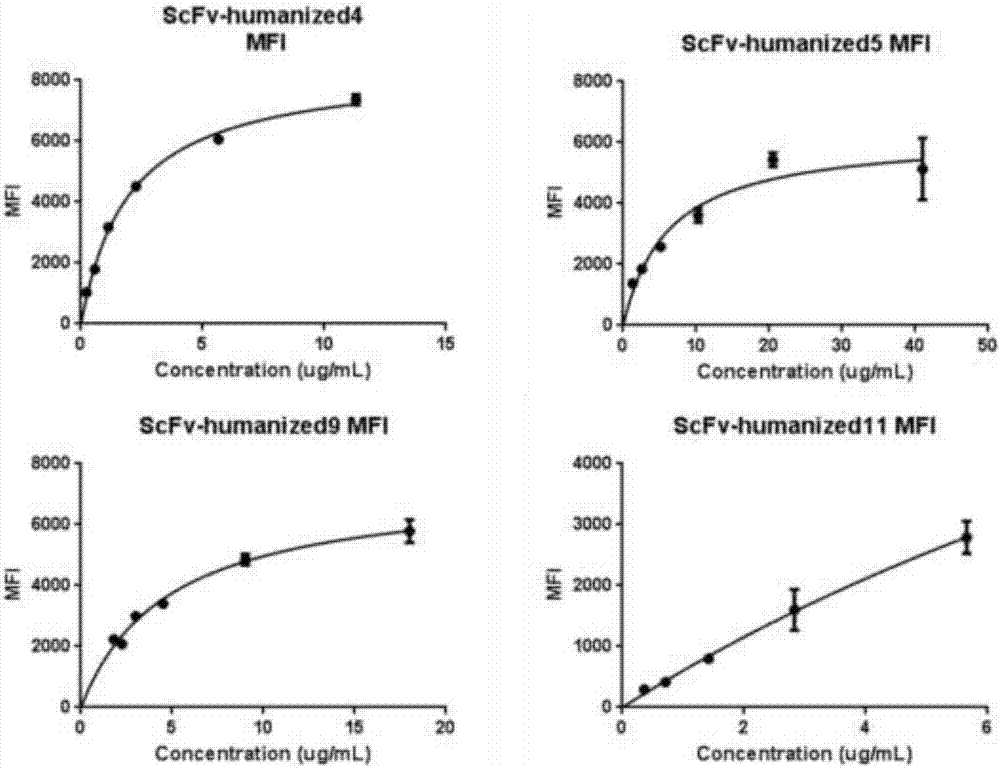
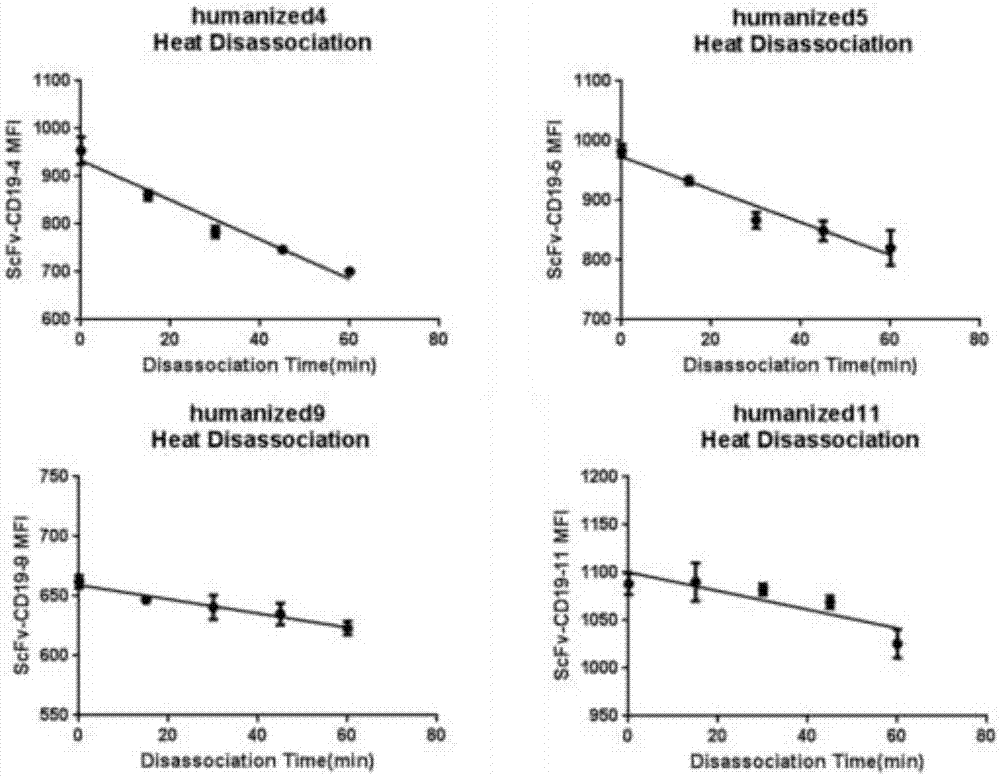
[0069] The monoclonal antibody scFV was labeled with a His tag and cloned into a plasmid vector and transfected into HEK293 cells. The cell supernatant was harvested through a 0.45um filter, and the filtrate was taken. Dilute with an equal volume of equilibration buffer and measure the pH. After ultrafiltration and concentration, use a 0.22μm syringe filter to sterilize and filter, aliquot, label and store in a -80°C refrigerator. Different humanized antibodies were incubated with CD19-positive cells Raji and CD19-negative cells K562 for 30 minutes, and then the excess antibodies were washed away. After staining with FITC-His antibody for 30 minutes, the unlabeled antibodies were washed away, and flow cytometric detection was performed. The results are shown in the table As shown in 2, there are four monoclonal antibodies that can recognize the positive cell Raji and do not re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com