Method for preparing azo dye with alkalescent arylamine serving as diazotization ingredient
An azo dye, weak alkaline technology, applied in azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of water or soil acidification, low utilization rate of sulfuric acid, ecological environment hazards, etc. The effect of avoiding pollution reprocessing and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
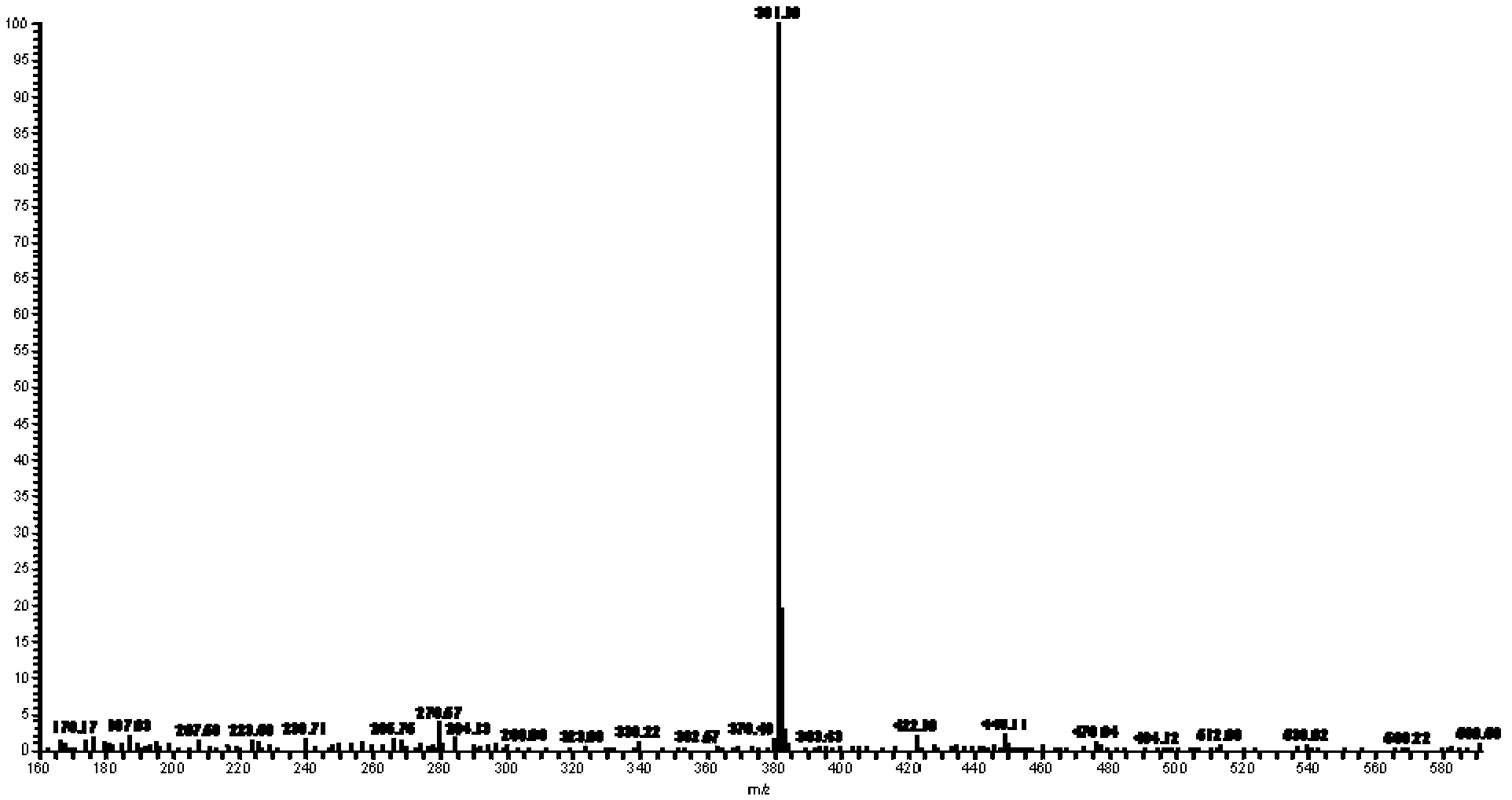
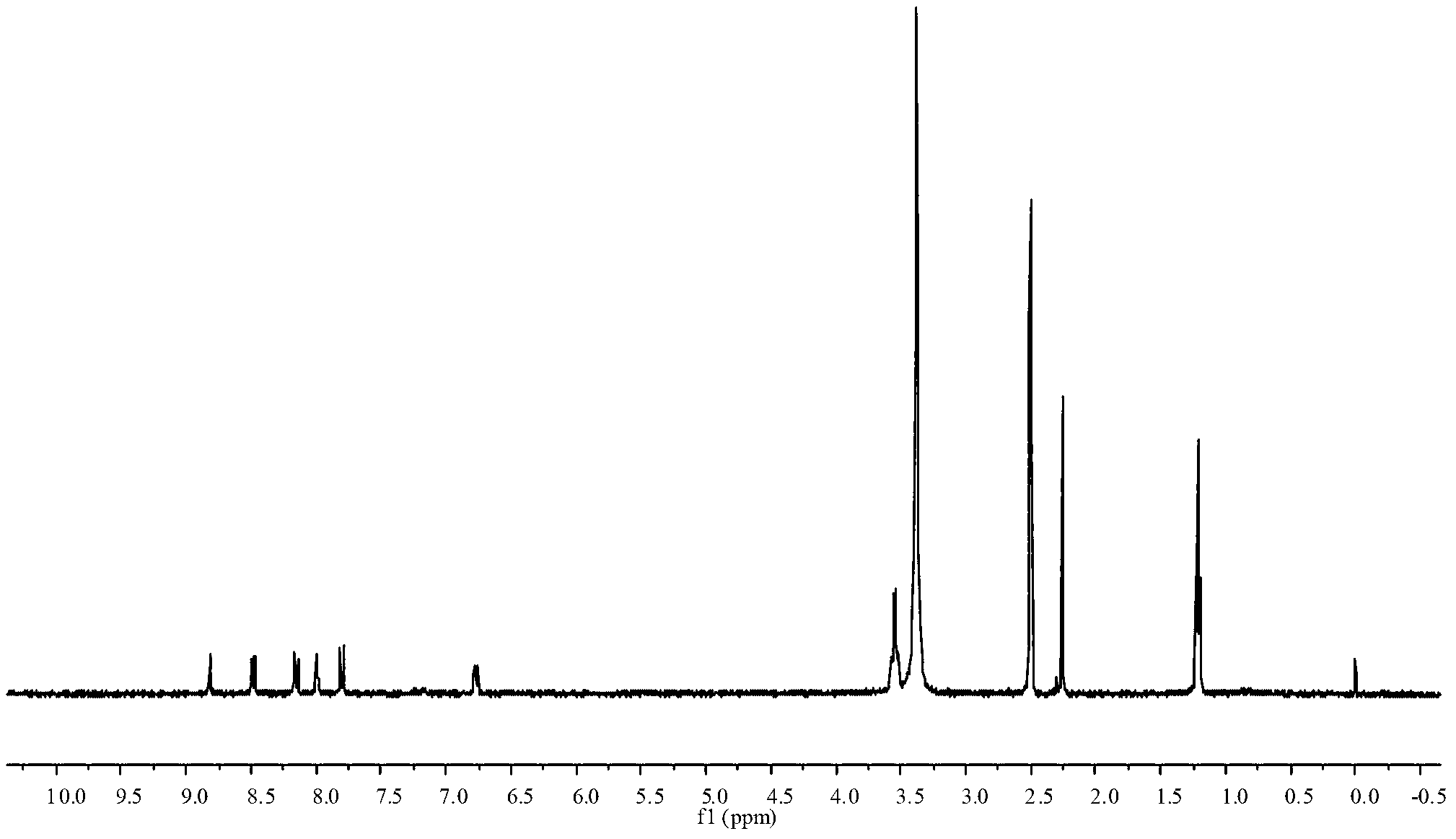
Image
Examples
Embodiment 1
[0026] 1st reaction
[0027] (1) Preparation of weakly basic arylamine diazonium salt solid: Add 40ml of ethyl acetate and 0.82g of 2-cyano-4-nitroaniline (diazo component) into a 100ml three-necked flask connected with electric stirring, Stir to dissolve, ice bath (0-5 ℃) 10min. Add 2.75g of 40% fluoboric acid and 5.0g of 98% concentrated sulfuric acid dropwise, and continue stirring for 10 minutes. Then 0.35 g of sodium nitrite was added, and the reaction was stirred vigorously for 1.5 h. Thin-layer chromatography detected that the reaction of the raw materials had been completed. Suction filtration at low temperature (0-5°C) to obtain a white crystalline solid, and the pale yellow filtrate is recycled in an ice bath (0-5°C).
[0028] (2) Coupling reaction: Add 1.03g of 3-(N,N-diethyl)aminoacetanilide (coupling component) into a 500ml beaker filled with 10ml of water, add a small amount of acid and stir to dissolve, ice bath (0 -5°C) 10min. Under vigorous stirring, crush...
Embodiment 2
[0046] The first reaction: except that the diazo component is 0.92g 2,4-dinitroaniline, and the addition amount of 40% fluoroboric acid is 1.65g, other additions and operations are the same as the first reaction in Example 1. This reaction obtained 1.51 g of solid dye, and the yield was 75.5%.
[0047] The 1st to 6th cycle reactions: the solid preparation process of weakly basic arylamine diazonium salts uses the filtrate of the previous cycle reaction, no need to add ethyl acetate, only 0.50g98% concentrated sulfuric acid is added, and the remaining diazo components , fluoboric acid and sodium nitrite addition and reaction operation are the same as the first reaction, and the coupling reaction is also the same as the first reaction. The amount of dyes obtained in the 1st to 6th cycle reactions were 1.71g, 1.77g, 1.76g, 1.82g, 1.64g and 1.69g respectively, and the yields were 85.5%, 88.5%, 88.0%, 91.0%, 82.0% respectively and 84.5%.
[0048] After 6 cycles of reaction, the d...
Embodiment 3
[0055] The first reaction: except that the diazo component is 1.21g 2-cyano-4-nitro-6-bromoaniline, and the addition of ethyl acetate is 60ml, other additions and operations are the same as the first reaction in Example 1 same. This reaction yielded 1.88 g of solid dye, with a yield of 82.1%.
[0056] The 1st to 4th cycle reactions: the solid preparation process of weakly basic arylamine diazonium salts uses the filtrate of the previous cycle reaction, no need to add ethyl acetate, only 0.50g of 98% concentrated sulfuric acid and 40% fluoboric acid are added The amount is 2.20g, and the addition amount and reaction operation of the remaining diazo components and sodium nitrite are the same as the first reaction, and the coupling reaction is also the same as the first reaction. The amount of dyes obtained from the first to the fourth cycle reactions were 2.03g, 1.99g, 1.92g and 1.88g, respectively, and the yields were 88.6%, 86.5%, 83.5% and 82.0%, respectively.
[0057] Afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com