Application of diallyl propyl flavonolignan in preparation of medicament for curing hepatitis B
A flavonoid lignan and allyl group substitution technology is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve the problems that new uses have not been effectively developed, and achieve large-scale production. Large-scale production, less pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
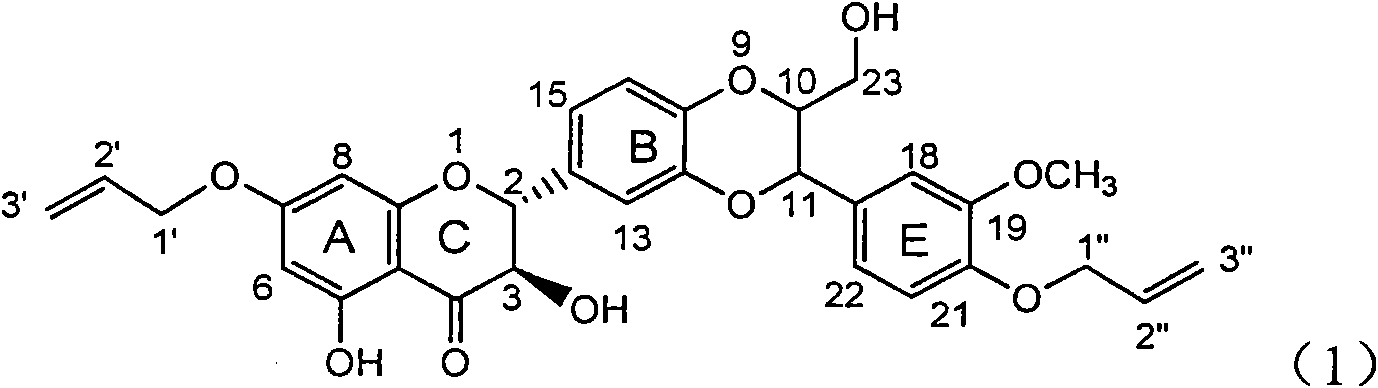
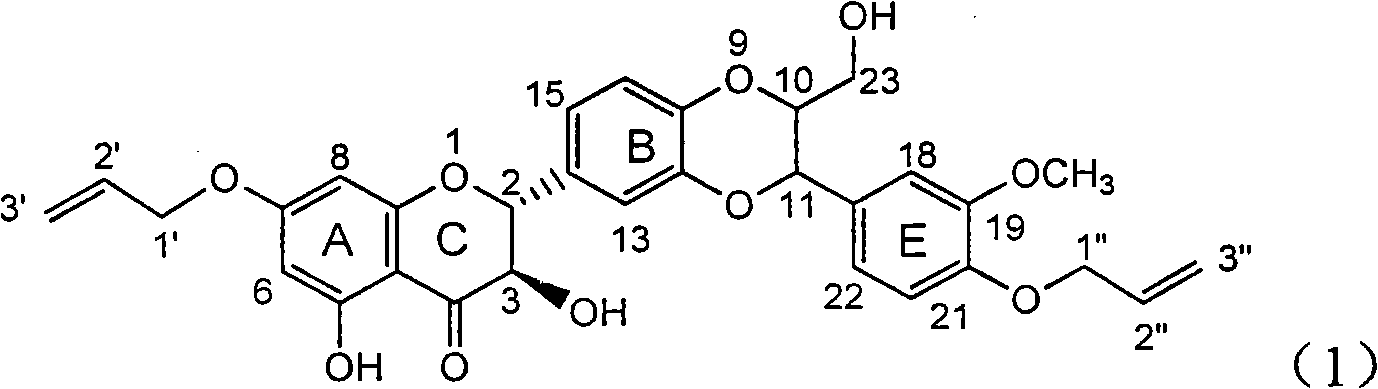
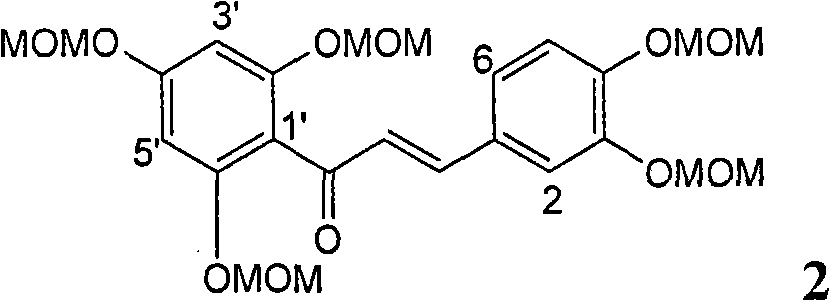
[0025] Example 1: Formula (1) compound 2,3-dihydro-2-[2,3-dihydro-3-(4-allyloxy-3-methoxyphenyl)-2-hydroxymethyl-1, Preparation of 4-benzodioxane-6-]-7-(allyloxy)-3,5-dihydroxy-4H-1-benzopyran-4-one
[0026] The present invention has prepared the flavonoid lignan compound shown in formula (1) by a de novo synthesis method. In addition to this method, it can also be used to directly carry out the final oxidative coupling reaction with commercially available silibinin and allyl bromide. The compound of formula (1) is generated in one step. The instruments and reagents used include:
[0027] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); (100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin laye...
Embodiment 2
[0044] Example 2: Inhibitory effect of compound of formula (1) on hepatitis B e antigen (HBeAg) secreted by HepG2.2.15 cells
[0045] 2.1 Cell culture:
[0046] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin, 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0047] 2.2 The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth was measured by MTT method:
[0048] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 cells / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 After 24 hours in an incubator with 100% relative humidity, add compound (1) diluted with medium, the concentration is 1000 μg / ml, 200 μg / ml, 40 μg / ml and 8 μg / ml, 200 μg / ml in each well microliter, each concentration was set up in triplicate, placed at 37°C, 5% CO 2 , cultivated i...
Embodiment 3
[0055] Example 3: Inhibition of the replication of hepatitis B virus deoxyribonucleic acid (HBV DNA) secreted by the compound of formula (1) to HepG2.2.15 cells
[0056] 3.1 Cell culture: the method is the same as in Example 2.
[0057] 3.2 The inhibitory effect of the flavonoid lignan compound represented by formula (1) on the growth of HepG2.2.15 cells was determined by MTT method: the method is the same as that in Example 2.
[0058] 3.3 The flavonoid lignan compounds shown in the assay formula (1) inhibit the replication of hepatitis B virus deoxyribonucleic acid (HBV DNA): get the HepG2.2.15 cells in the logarithmic growth phase, and use the culture medium to dilute the cells to 1 ×10 5 cells / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2After cultivating in an incubator with 100% relative humidity for 24 hours, add the flavonoid lignan compound shown in the formula (1) diluted with the medium, the concentration is respectively 5 microgram...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com