Multipotent Adult Stem Cells And Uses of Multipotent Adult Stem Cells To Treat Inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
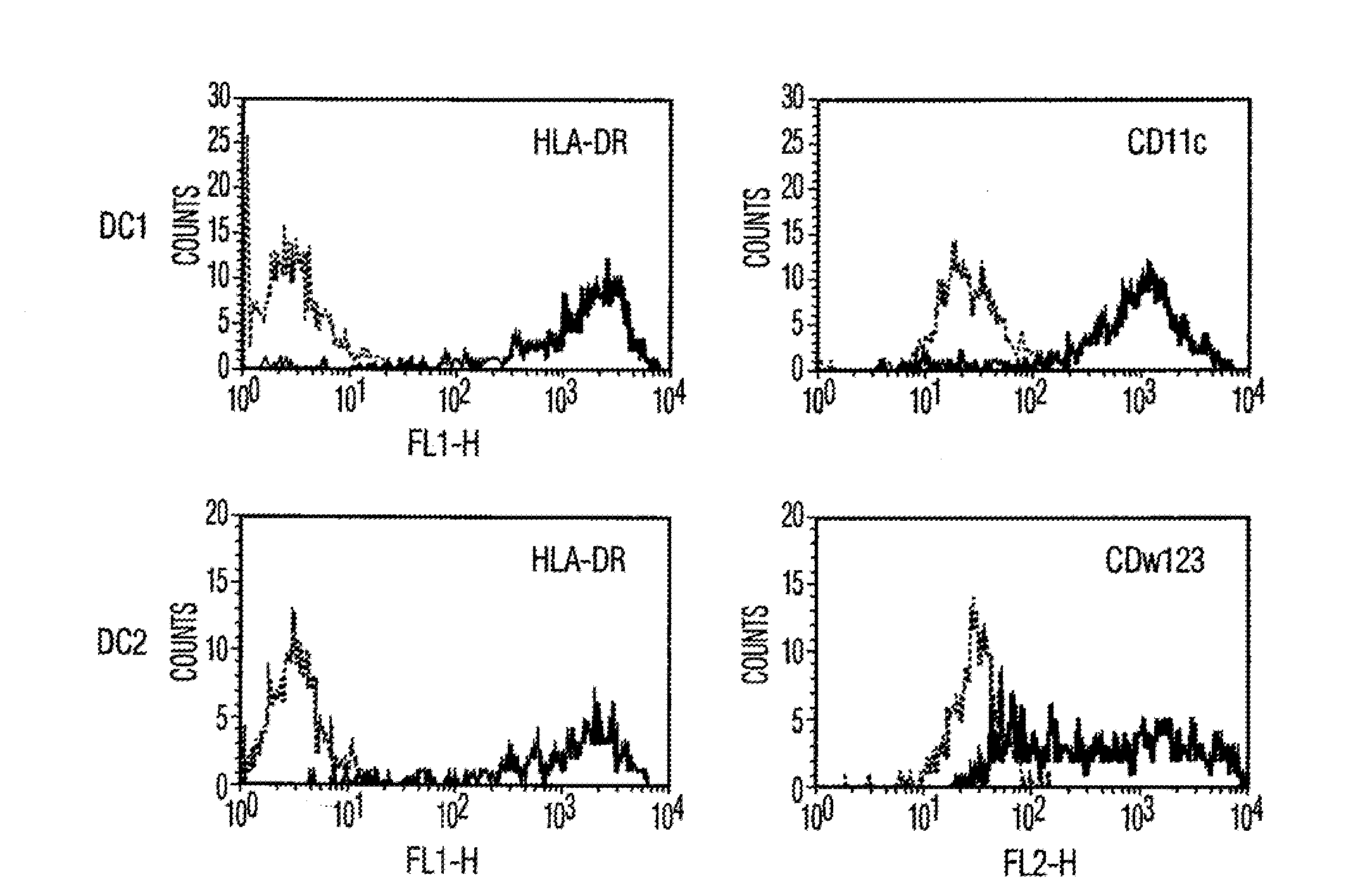
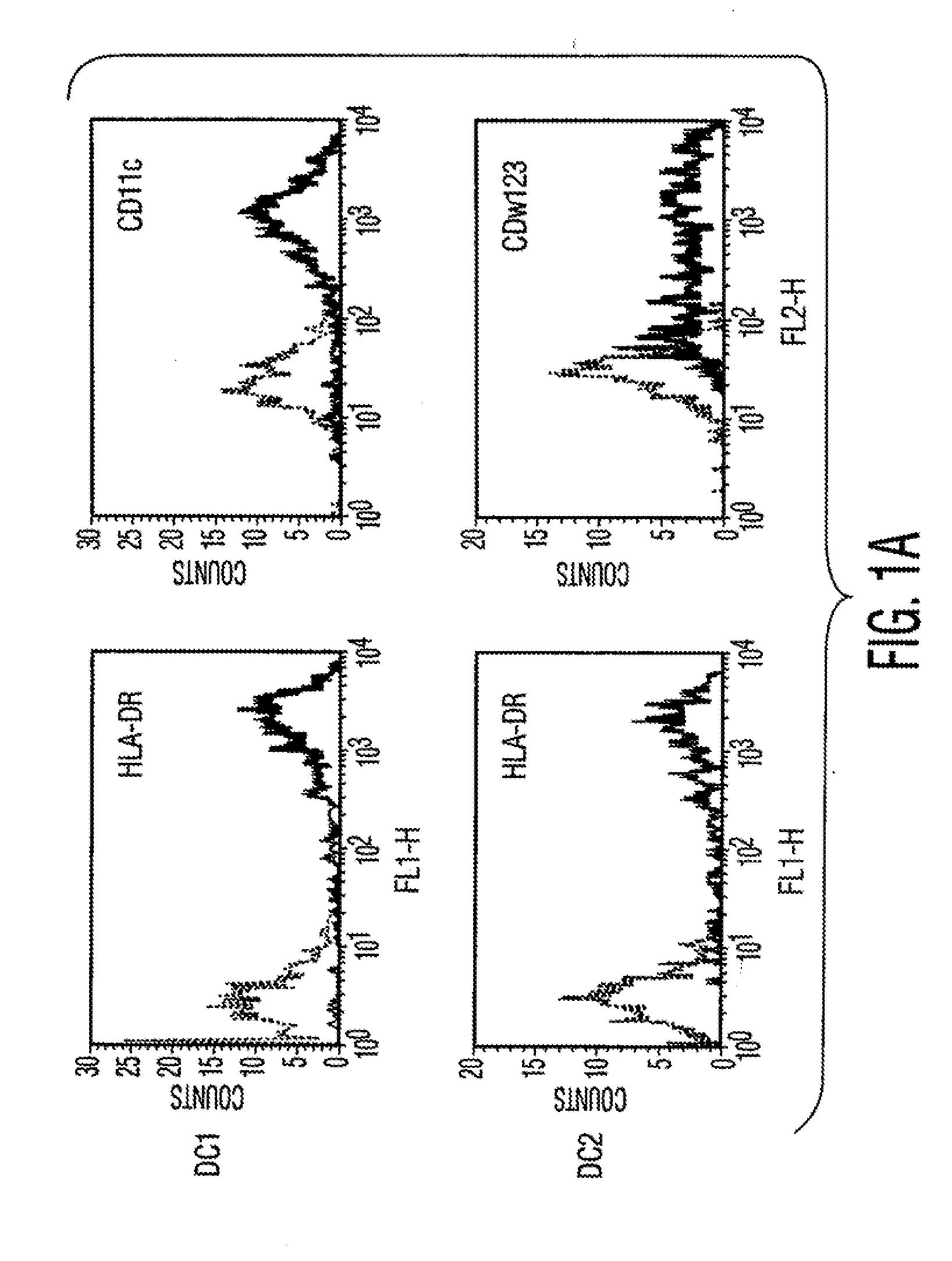
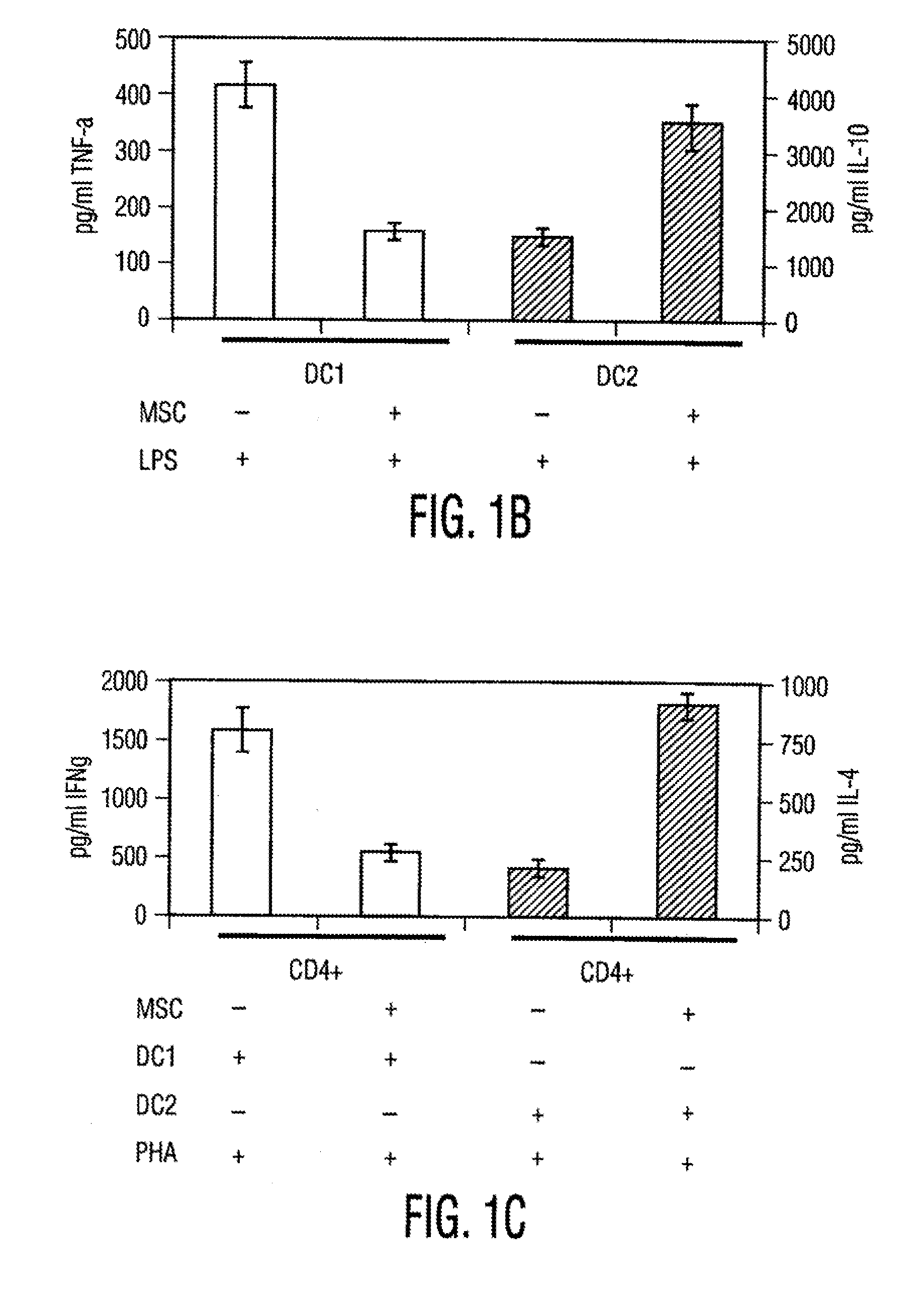
[0106]Materials and Methods Culture of human MSCs. Human MSCs were cultured as described by Pittenger et al., Science, Vol. 284, pg. 143 (1999). Briefly, marrow samples were collected from the iliac crest of anonymous donors following informed consent by Poietics Technologies, Div of Cambrex Biosciences. MSCs were cultured in complete Dulbecco's Modified Eagle's Medium-Low Glucose (Life Technologies, Carlsbad, Calif.) containing 1% antibiotic-antimyotic solution (Invitrogen, Carlsbad, Calif.) and 10% fetal bovine serum (FBS, JRH BioSciences, Lenexa, Kans.). MSCs grew as an adherent monolayer and were detached with trypsin / EDTA (0.05% trypsin at 37° C. for 3 minutes). All MSCs used were previously characterized for multilineage potential and retained the capacity to differentiate into mesenchymal lineages (chondrocytic, adipogenic, and osteogenic) (Pittenger, et al., Science, Vol. 284, pg. 143 (1999)).
[0107]Isolation of Dendritic cells. Peripheral blood mononuclear cells (PBMCs) were...
example 2
[0125]Mesenchymal stem cells were given to a 33-year-old female patient suffering from severe Grade IV gastrointestinal graft-versus-host disease (GVHD). The patient was refractory to all other GVHD treatments. Endoscopic views of the patient's colon showed areas of ulceration and inflammation prior to treatment. Histology of the patient's colon showed that the graft-versus-host disease had destroyed the vast majority of the patient's intestinal crypts, prior to treatment.
[0126]The patient was given an intravenous infusion of allogeneic mesenchymal stem cells in 50 ml of Plasma Lyte A (Baxter) in an amount of 3×106 cells per kilogram of body weight.
[0127]The patient was evaluated at two weeks post-infusion. At two weeks post-infusion, an endoscopic view of the patient's colon showed that the areas of inflammation and ulceration visible prior to treatment were resolved. In addition, a biopsy of the patient's colon showed significant regeneration of intestinal crypts. Thus, the admini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com