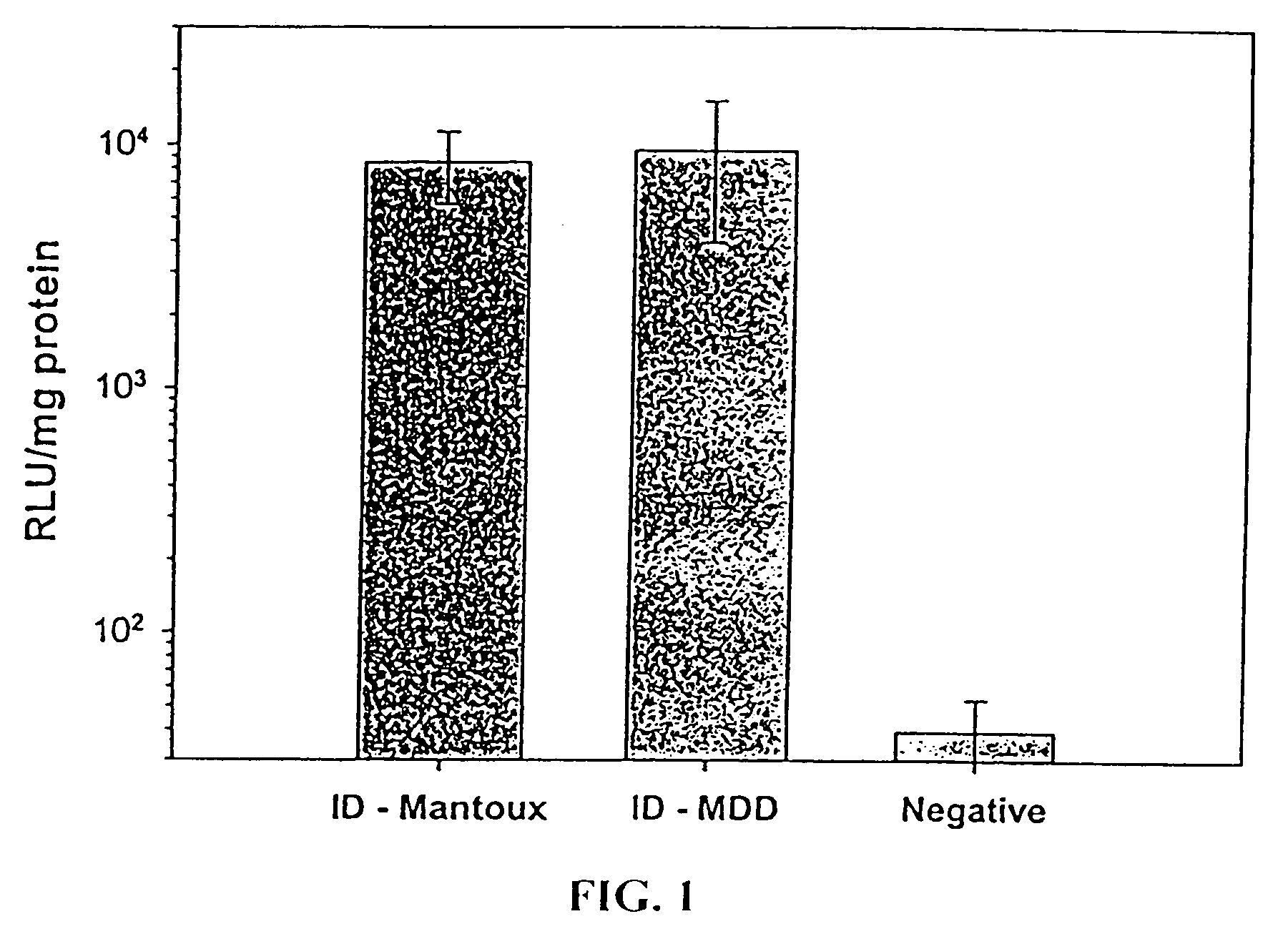
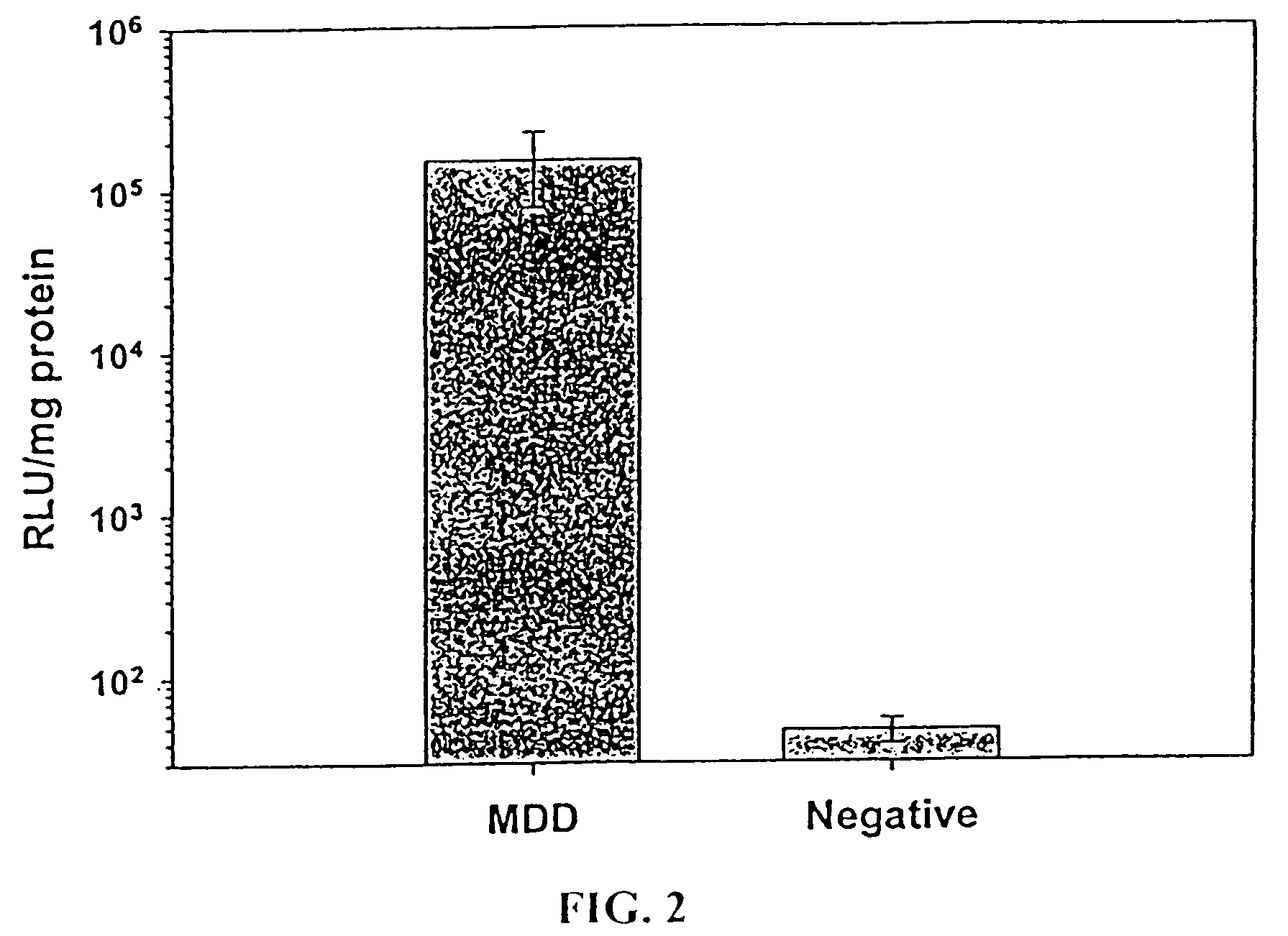
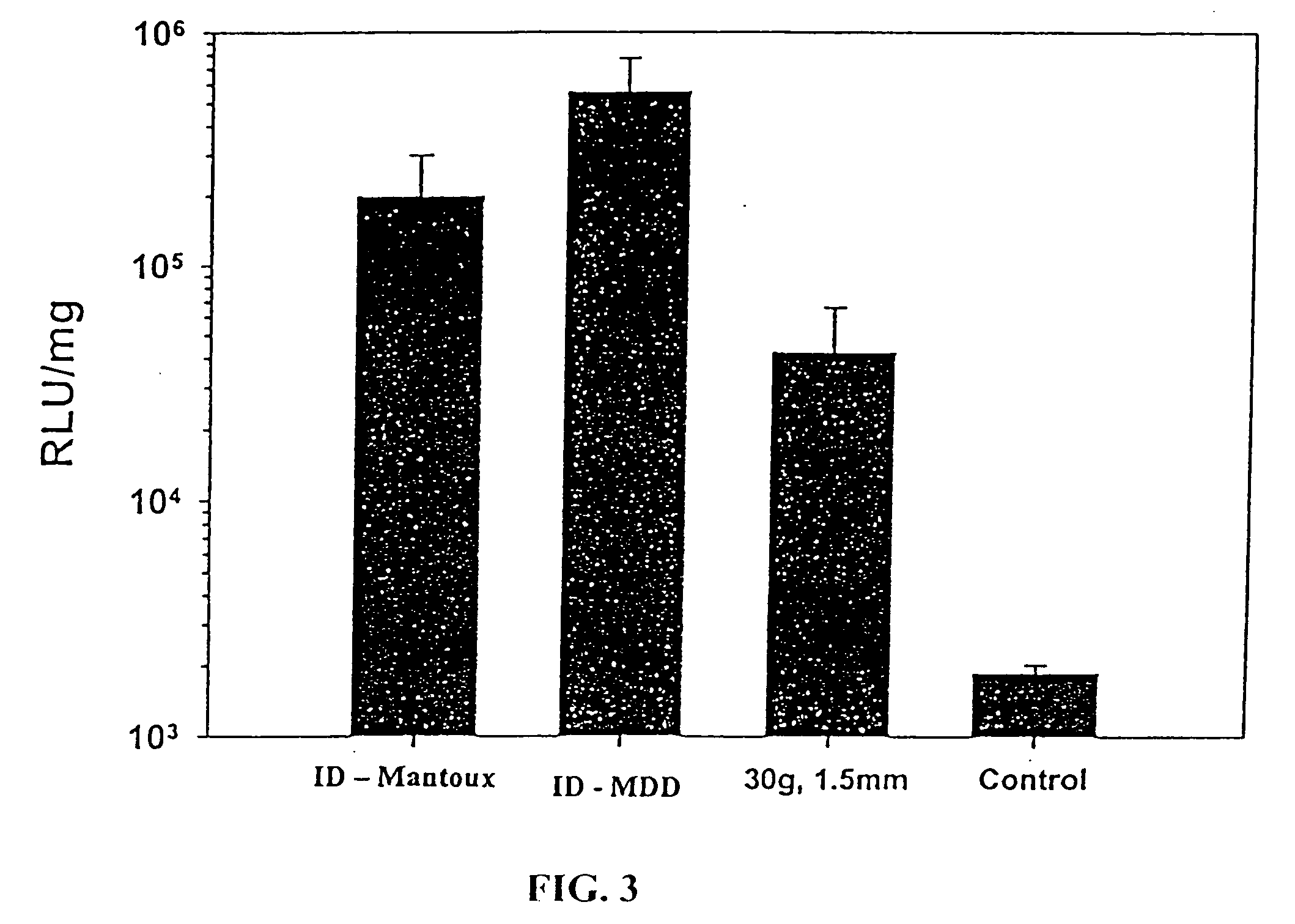
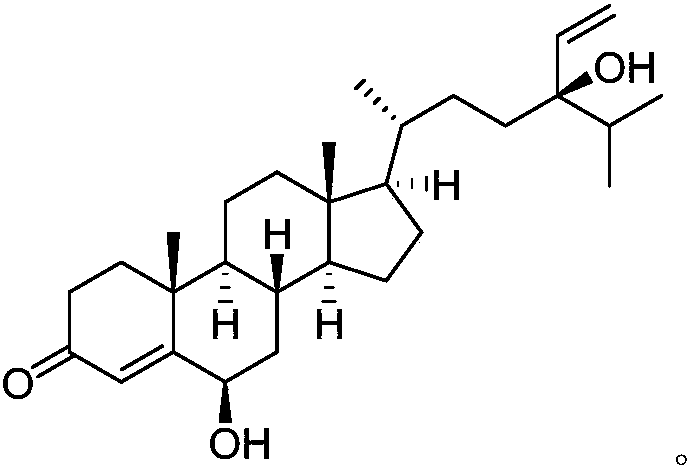
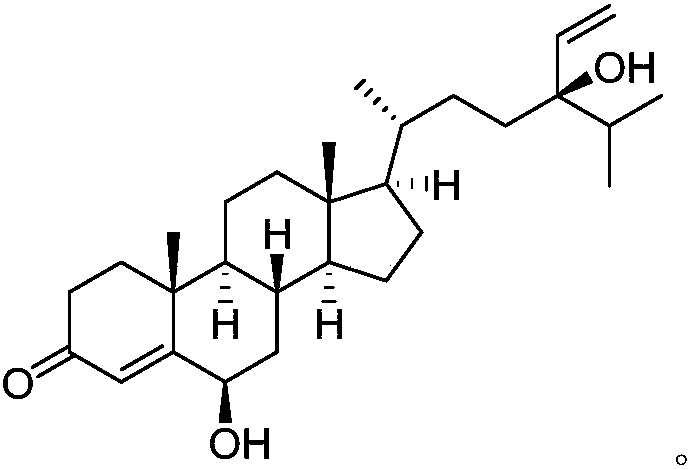
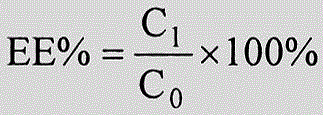Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Intramuscular route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intramuscular (also IM or im) injection is the injection of a substance directly into muscle. In medicine, it is one of several alternative methods for the administration of medications (see route of administration).
Liquid pharmaceutical composition for the delivery of active ingredients
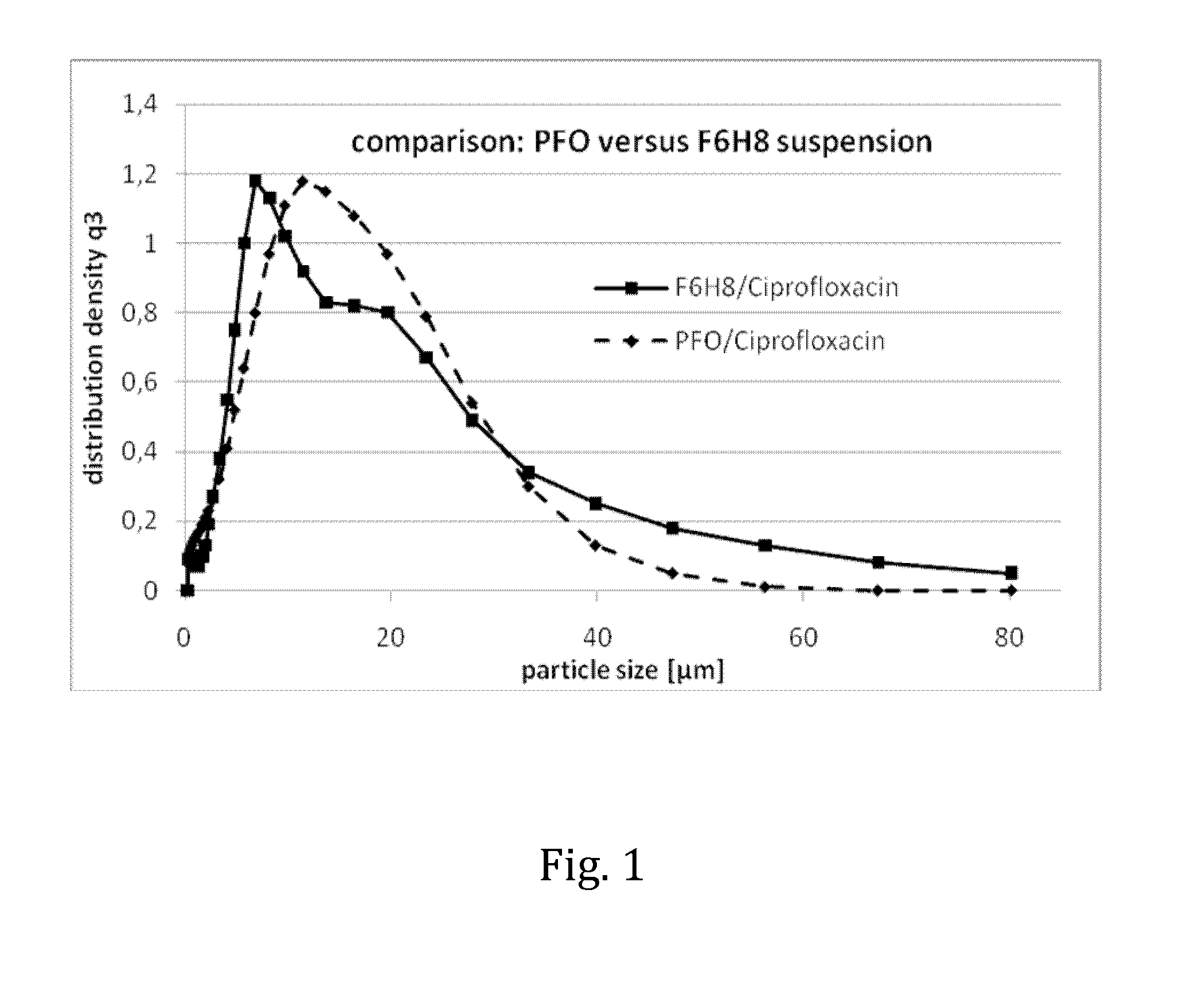
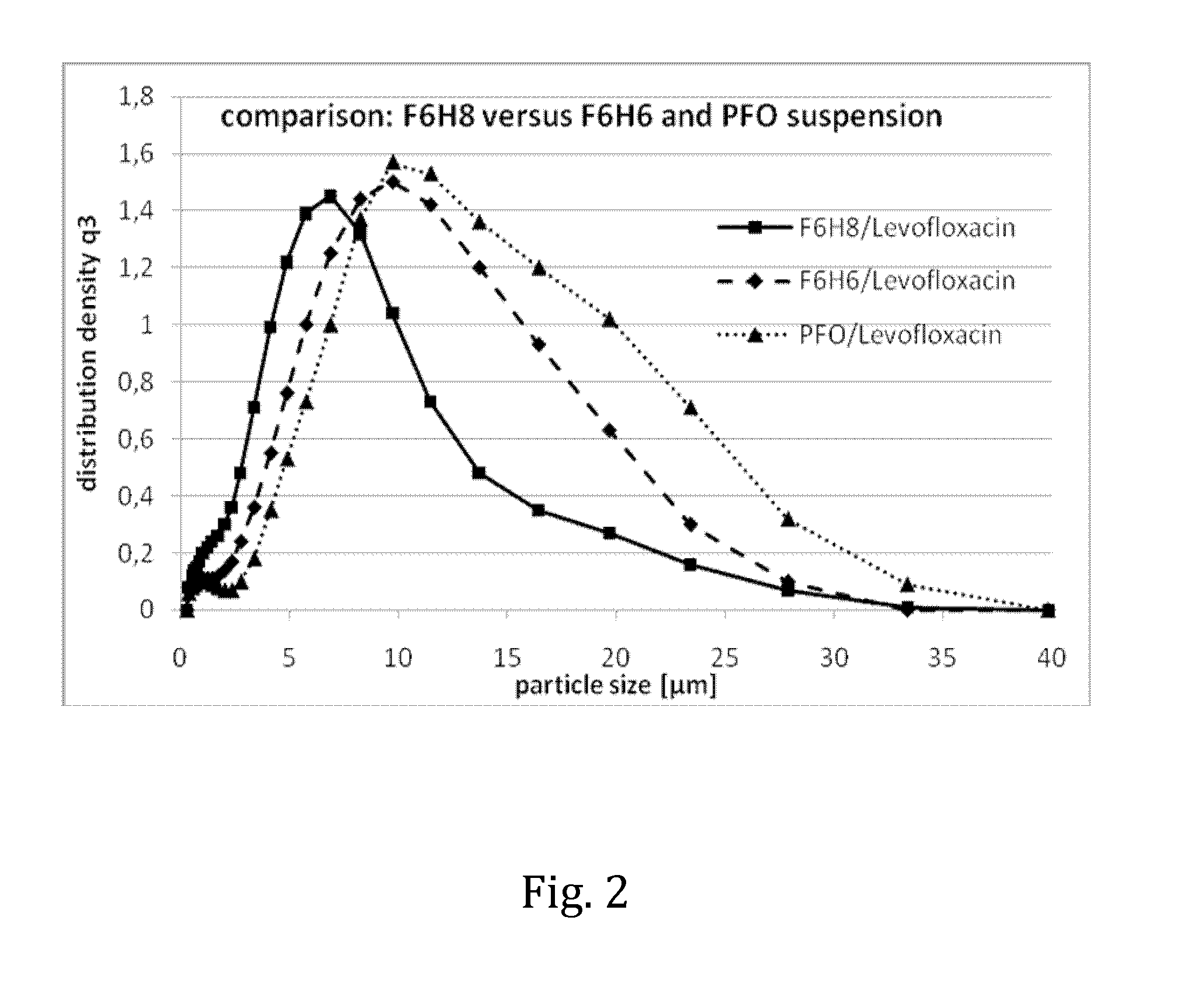
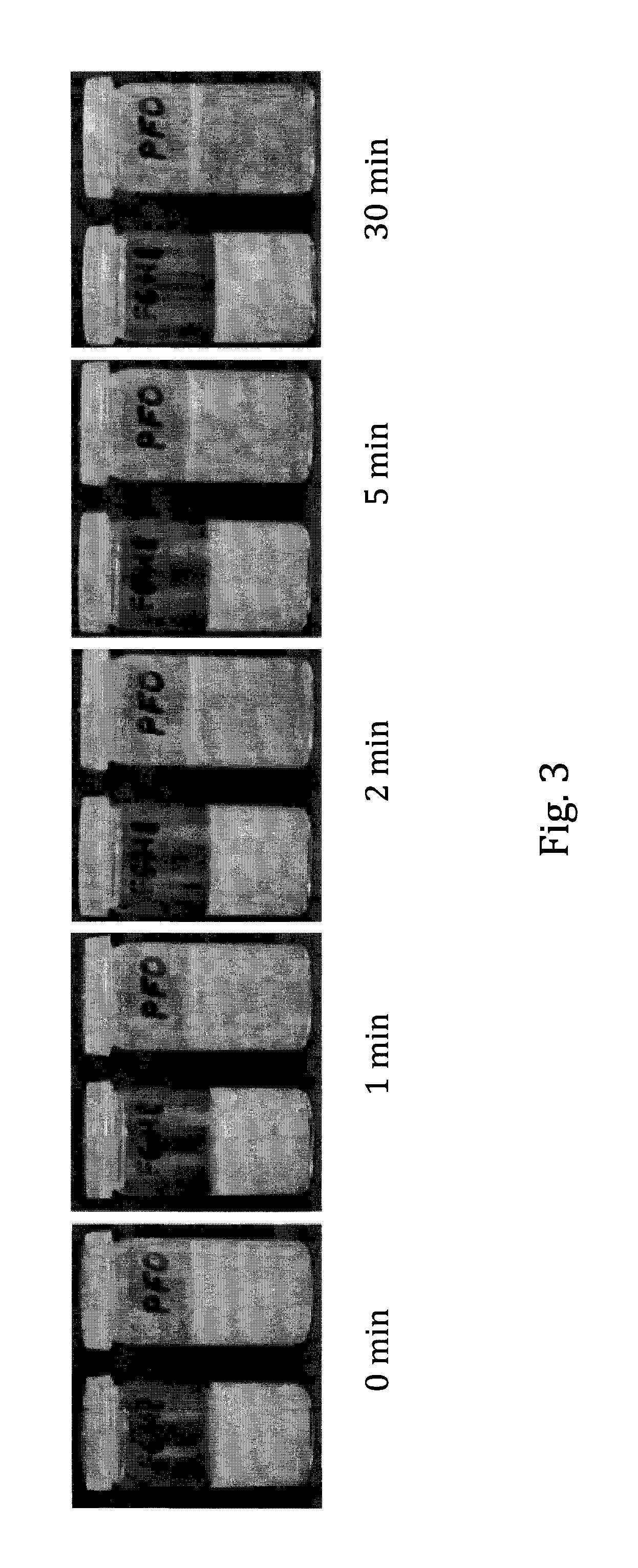
The invention provides novel pharmaceutical compositions based on semifluorinated alkanes which are useful as carriers for a broad range of active ingredients. Preferred active ingredients include poorly water-soluble and / or hydrolytically sensitive drug substances. The compositions are designed as suspensions and have superior physical properties which make them highly useful as pharmaceutical delivery systems. The compositions may be administered topically into the eye, or injected via the subcutaneous or intramuscular route. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Stabilised protein compositions based on semifluorinated alkanes
ActiveUS20140369993A1Stable dispersion and suspensionRemarkable stabilising effectPeptide/protein ingredientsCalcitoninsAlkaneProtein
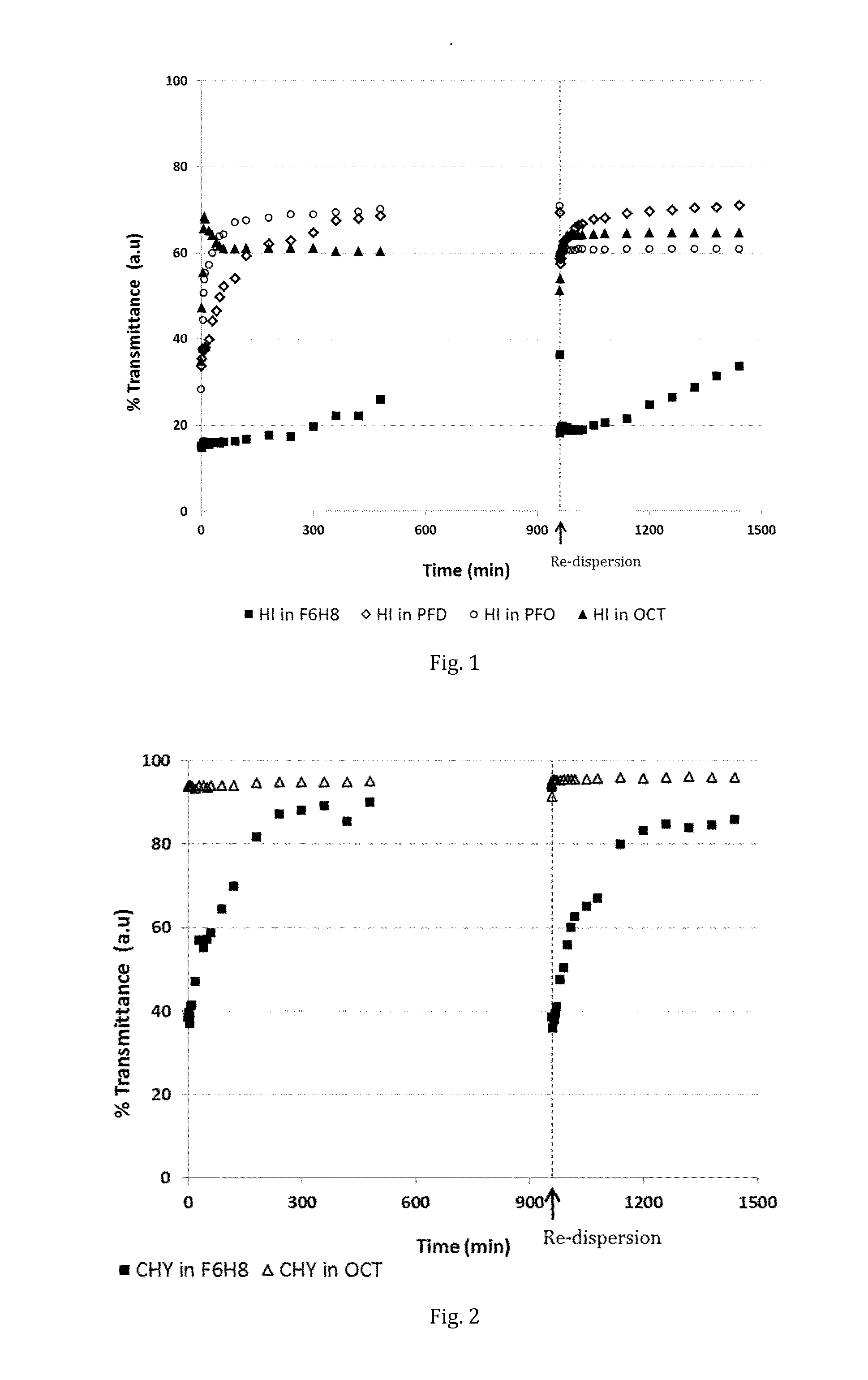
The invention provides novel compositions of bioactive polypeptides and proteins with improved stability and shelf-life. The compositions are based on liquid vehicles selected from semifluorinated alkanes. These vehicles are remarkably effective in protecting polypeptides and proteins from degradation and / or aggregation. The compositions are useful for topical administration, e.g. into an eye, or by parenteral injection, e.g. via the subcutaneous or intramuscular route.
Owner:NOVALIQ GMBH
Liquid pharmaceutical composition for the delivery of active ingredients
The invention provides novel pharmaceutical compositions based on semifluorinated alkanes which are useful as carriers for a broad range of active ingredients. Preferred active ingredients include poorly water-soluble and / or hydrolytically sensitive drug substances. The compositions are designed as suspensions and have superior physical properties which make them highly useful as pharmaceutical delivery systems. The compositions may be administered topically into the eye, or injected via the subcutaneous or intramuscular route. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Factor VII polypeptides for preventing formation of inhibitors in subjects with haemophilia
InactiveUS20050032690A1Inhibition formationImprove stabilityOrganic active ingredientsFactor VIIFactor VIIaBlood coagulation factor VIII
The invention provides a method for preventing formation of inhibitors to blood coagulation factor VIII or factor IX in a subject having haemophilia, the method comprising administering (via intravenous, subcutaneous, intradermal, or intramuscular routes) to a previously untreated subject an effective dosage of factor VIIa or a factor VII-related polypeptide.
Owner:NOVO NORDISK AS
Stabilised protein compositions based on semifluorinated alkanes
ActiveUS9757460B2Stable dispersion and suspensionRemarkable stabilising effectPeptide/protein ingredientsPharmaceutical delivery mechanismAlkaneProtein composition
The invention provides novel compositions of bioactive polypeptides and proteins with improved stability and shelf-life. The compositions are based on liquid vehicles selected from semifluorinated alkanes. These vehicles are remarkably effective in protecting polypeptides and proteins from degradation and / or aggregation. The compositions are useful for topical administration, e.g. into an eye, or by parenteral injection, e.g. via the subcutaneous or intramuscular route.
Owner:NOVALIQ GMBH
Intradermal delivery of vacccines and therapeutic agents
InactiveUS20060018877A1Improve responseEfficacious improved responsivenessSsRNA viruses negative-senseBiocideVaccine deliveryIntramuscular route
The present invention relates to methods and devices for administration of vaccines and therapeutic agents into the intradermal layer of the skin. The methods of the present invention elicit increased humoral and / or cellular response as compared to conventional vaccine delivery methods, e.g., intramuscular route. Furthermore, the methods of the present invention facilitate induction of an immune response by an amount of vaccine which is otherwise insufficient for inducing an immune response when delivered via conventional vaccine routes, e.g., intramuscular route.
Owner:BECTON DICKINSON & CO
Sustained-release micro-spheres preparation containing recombined erythropoietin and preparation method and use thereof
InactiveCN101507712AImprove stabilityFlat surfacePeptide/protein ingredientsGranular deliveryFreeze-dryingBiocompatibility Testing
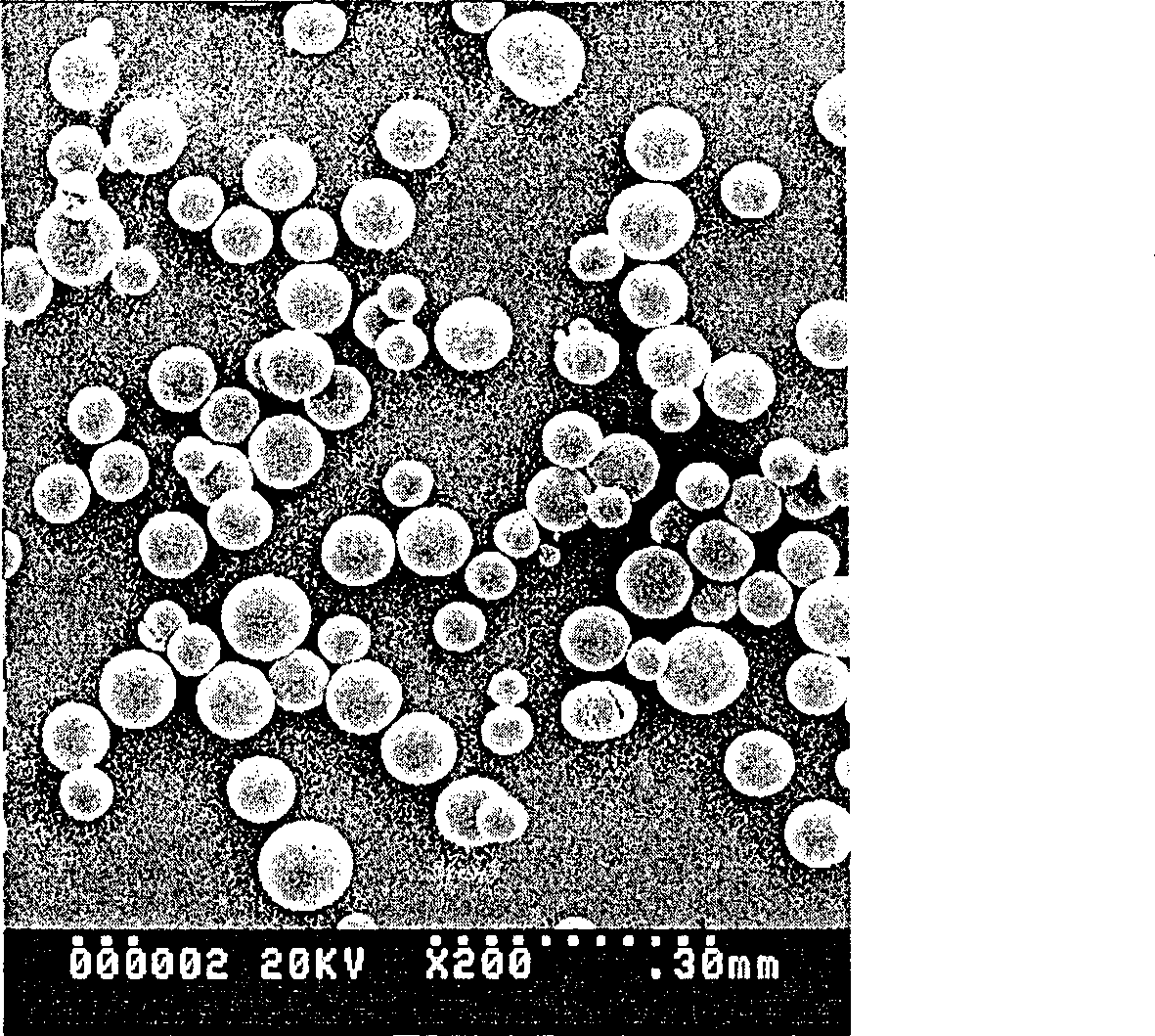
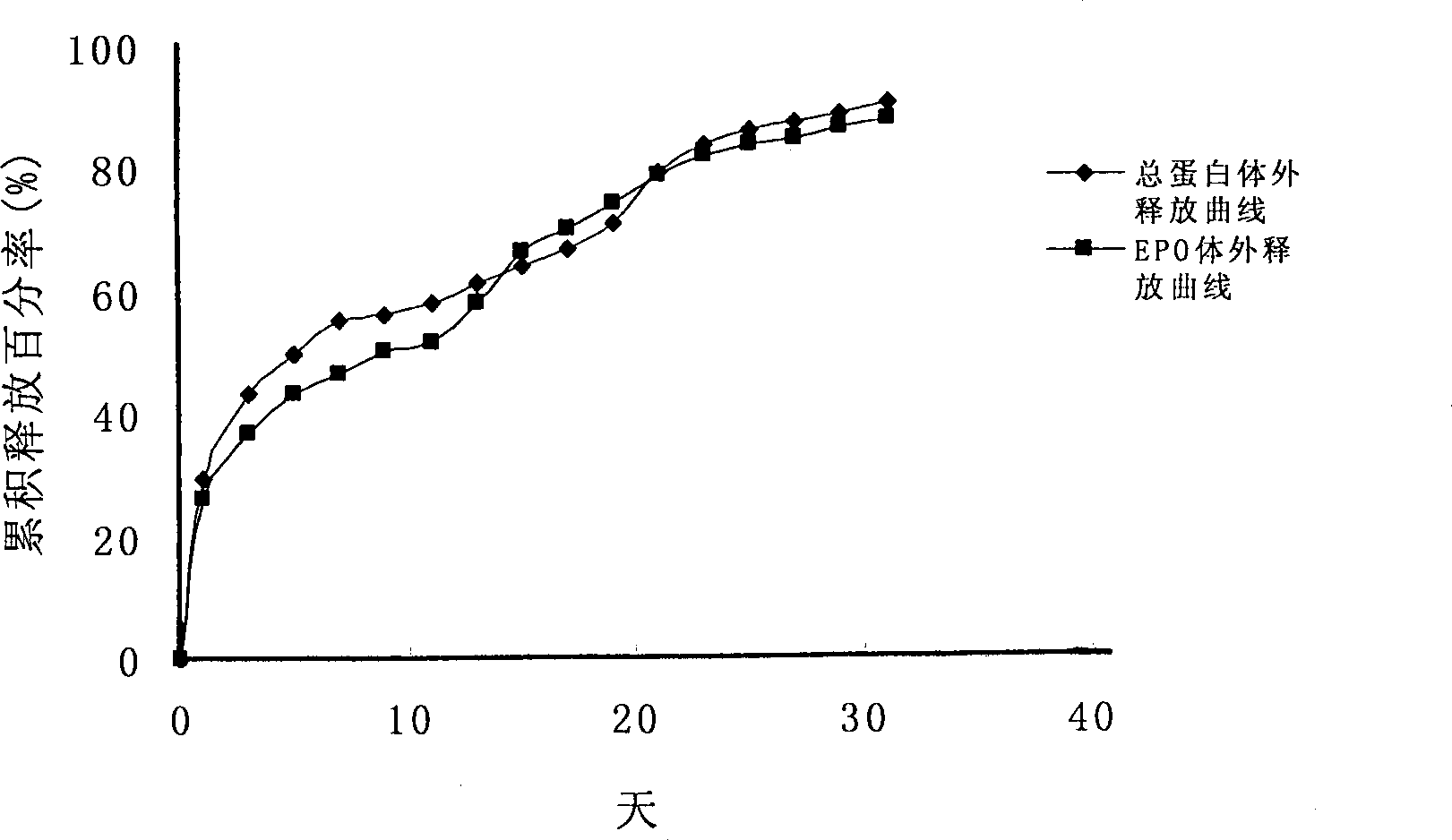
The invention discloses a slow-release microsphere preparation containing a recombined human haemopoietin. The slow-release microsphere preparation is prepared by the S / O / W compound emulsion solvent volatilization method. The preparation method comprises that: firstly, by the freeze-drying method, a micro particle containing the human serum albumin and the recombined human haemopoietin is prepared; secondly, by using a biodegradable high molecular material of lactic acid-glycollic acid block copolymer as a carrier material, the micro particle containing the human serum albumin and the recombined human haemopoietin is encapsulated; and thirdly, the lactic acid-glycollic acid block copolymer slow-release microsphere preparation containing the recombined human haemopoietin is prepared. The microsphere of the invention has the advantages of smooth surface, uniform appearance, regular size and no adhesion, the average particle size is between 70 and 105mu m; moreover, the microsphere is high in drug-carrying quantity and encapsulating rate, and the in vitro slow release period is more than 30 days. The obtained slow-release microsphere preparation is good in biocompatibility and can be used for non-intravenous drug administration such as hypodermic drug administration and intramuscular drug administration, and when used as a drug preparation for treating renal anemia, the slow-release microsphere preparation can improve the hematocrit of a patient.
Owner:HEBEI NORMAL UNIV
Compound of losartan compound or its medical salt and calcium channel blocker or its medical salt
InactiveCN101347427ALittle side effectsGood curative effectOrganic active ingredientsCardiovascular disorderCarboxylic acidLosartan
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
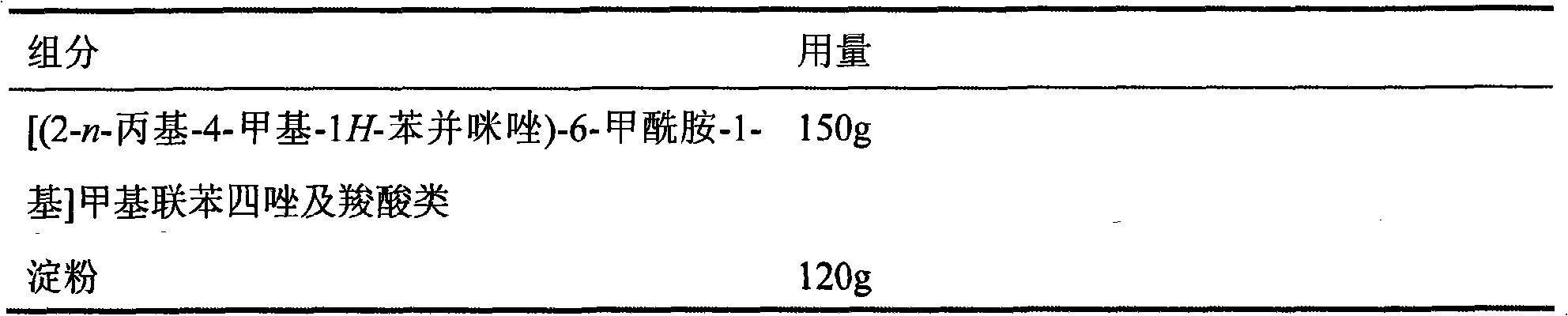
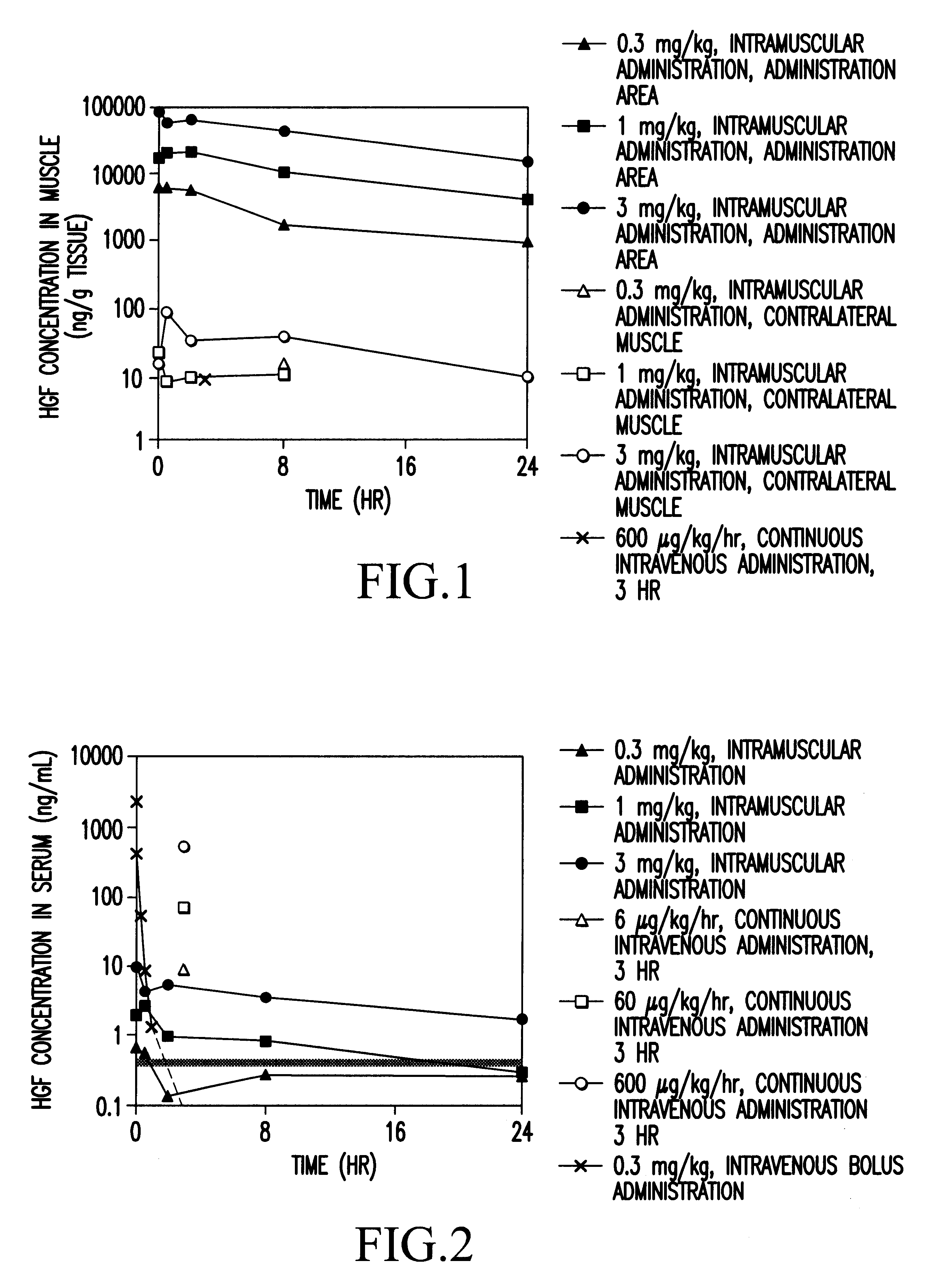
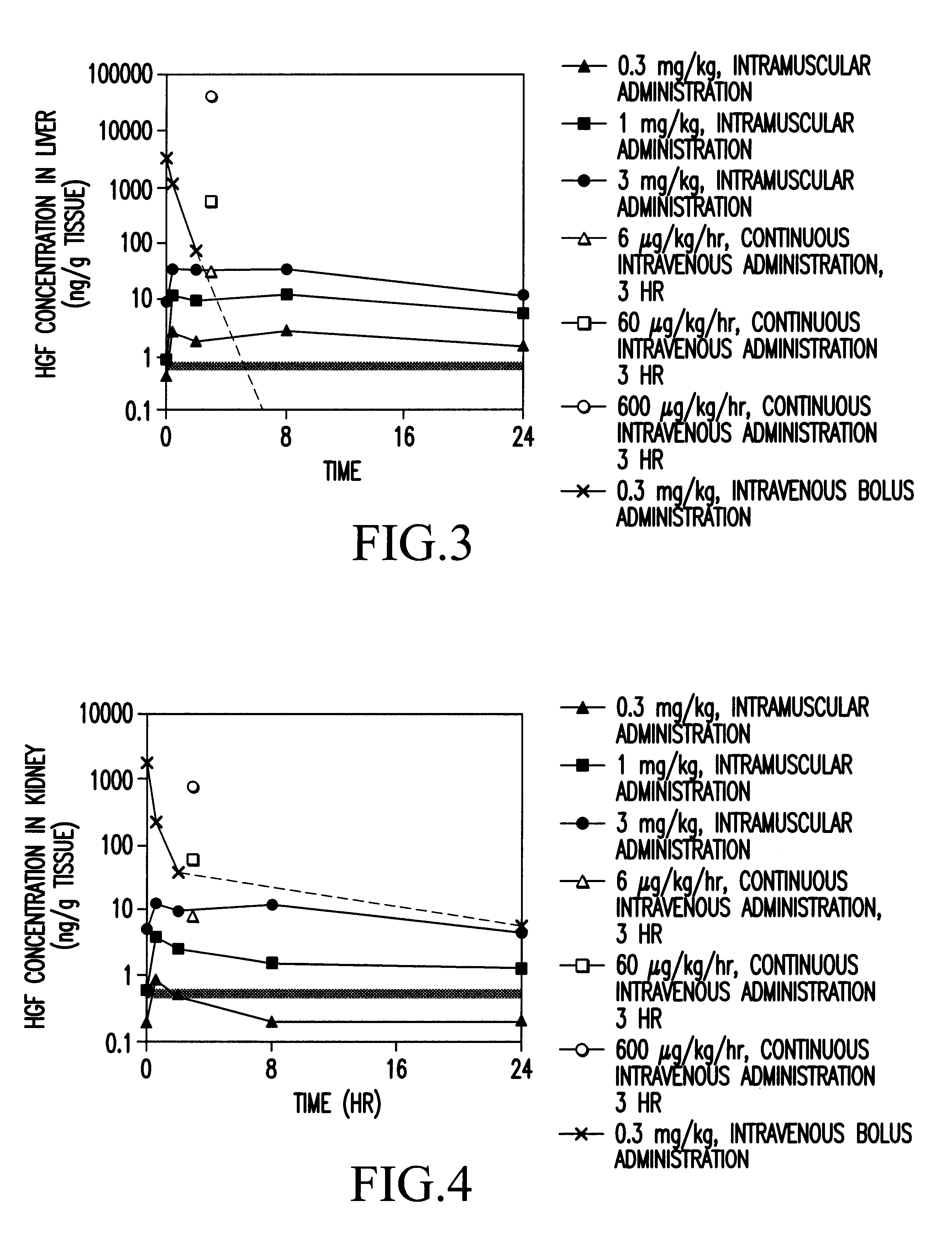
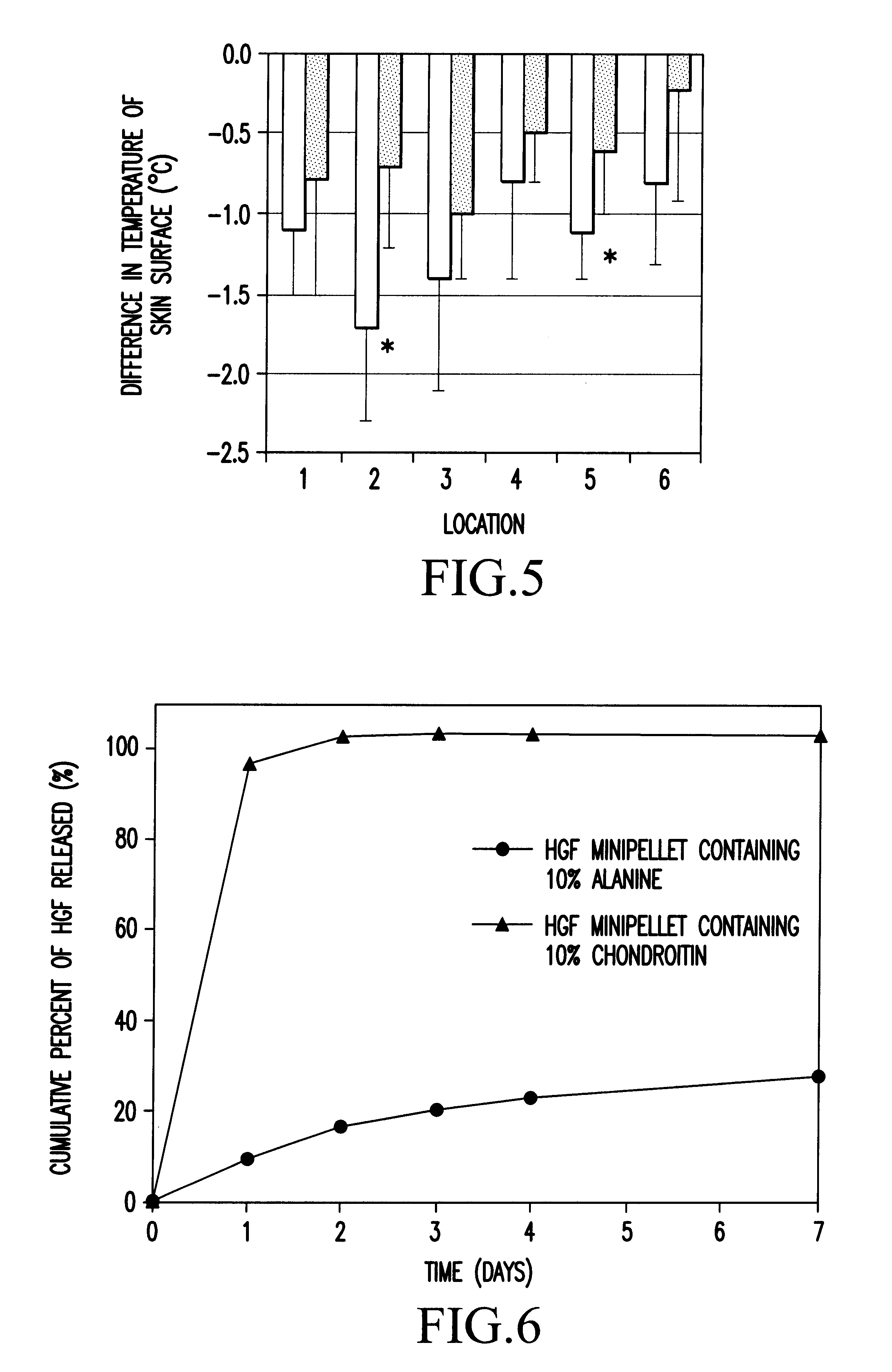
Method of treating ischemic disease by intramuscular administration of Hepatocyte growth factor
InactiveUS6887477B1Improvement of I/N ratioPromote blood circulationPeptide/protein ingredientsPeptide sourcesVeinHalf-life
Preparations to be administered for treating or preventing ischemic diseases or arterial diseases which contain Hepatocyte growth factor (HGF) as an active ingredient. The preparations provide such effect that HGF concentration in an affected region to which HGF was administered is maintained, a half-life is longer, a dose can be reduced, and other organs except the affected region are less affected in comparison with intravenous administration.
Owner:NAKAMURA TOSHIKAZU
Factor VII Polypeptides for Preventing Formation of Inhibitors in Subjects with Haemophilia
InactiveUS20110059894A1Reduce riskFactor VIIPeptide/protein ingredientsFactor VIIaBlood coagulation factor VIII
The invention provides a method for preventing formation of inhibitors to blood coagulation factor VIII or factor IX in a subject having haemophilia, the method comprising administering (via intravenous, subcutaneous, intradermal, or intramuscular routes) to a previously untreated subject an effective dosage of factor VIIa or a factor VII-related polypeptide.
Owner:NOVO NORDISK AS
Chinese traditional medicine compound, method for preparing the same and use thereof
InactiveCN101172143AInhibition of proliferation differentiationPrevent recurrenceAntineoplastic agentsPlant ingredientsHard CapsuleStomach cancer
The invention provides a Chinese traditional medicine compound prescription, and a preparation method and the purpose thereof, which mainly provides the Chinese traditional medicine compound prescription consisting of evodia, marsdenia tenacissima, climbing nightshade, oriental biter weed, Chinese fevervine, spatholobus stem, honey-suckle stem, wild grapevine, sargentg loryvine, Radix sophora flavescens, Paris Rhizome, licorice, etc. The preparation method comprisesthe following steps: step one, the alcohol reflux or the warm immersion abstraction of the evodia and the climbing nightshade, step two, the water extract-ethanol precipitation of gruffs and the rest medicinal materials, and step three, the blending of aqueous extract and ethanol extract. The invention provides the purposes of the compound prescription and the extract for the medicines of curing lung cancer, liver cancer, stomach cancer, rectal cancer, oophoroma, cervical carcinoma, prostatic cancer, bone cancer and other cancers, and simultaneously the invention also provides all administration forms including oral administration, rectum administration, intramuscular administration, vena administration, nasal cavity administration, vagina administration, subcutaneous administration, etc., and all preparation forms accepted by pellet, hard capsule, soft capsule, granula, oral liquid, syrup and pharmacy.
Owner:南京宇道科技开发有限公司
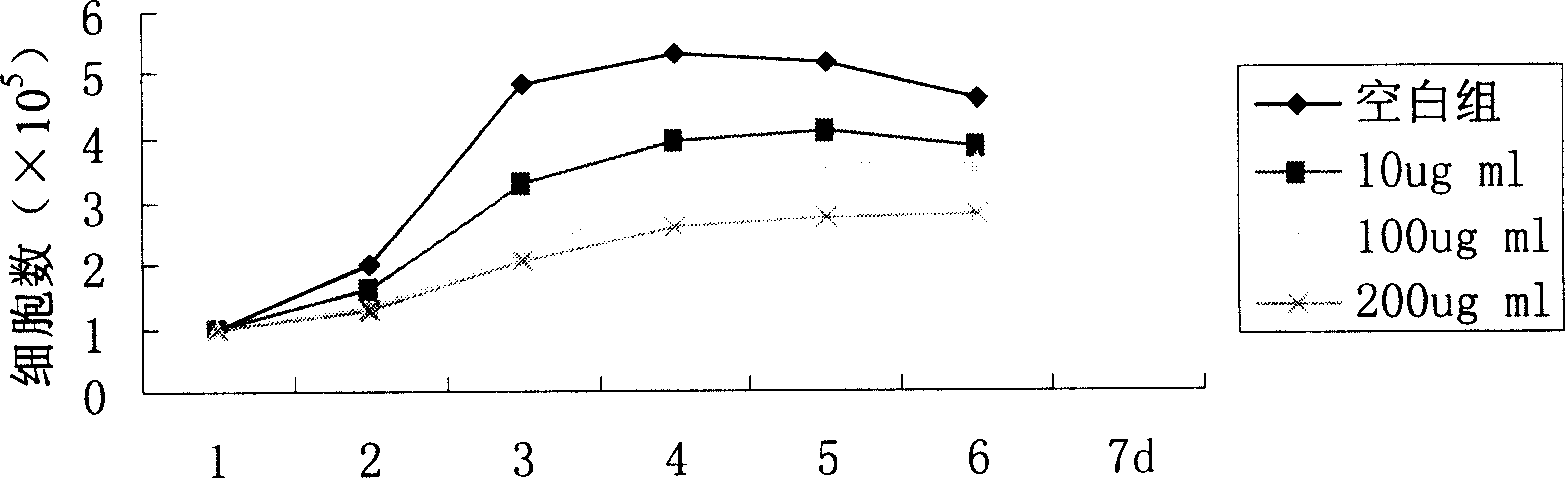
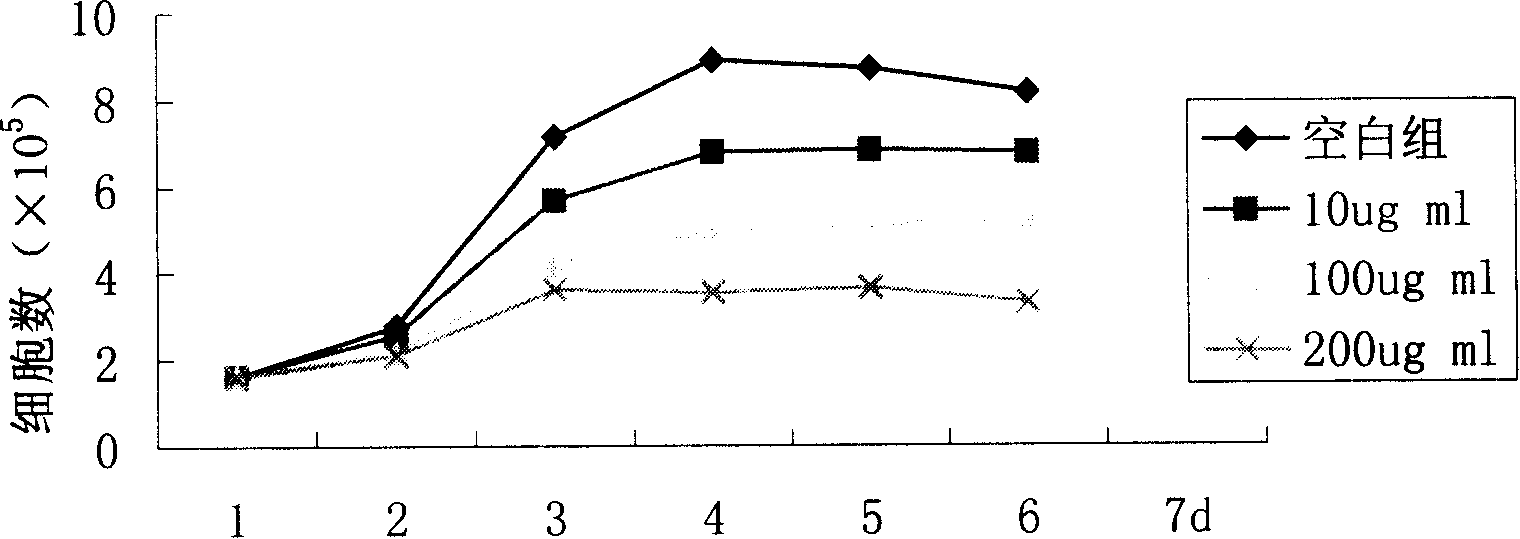
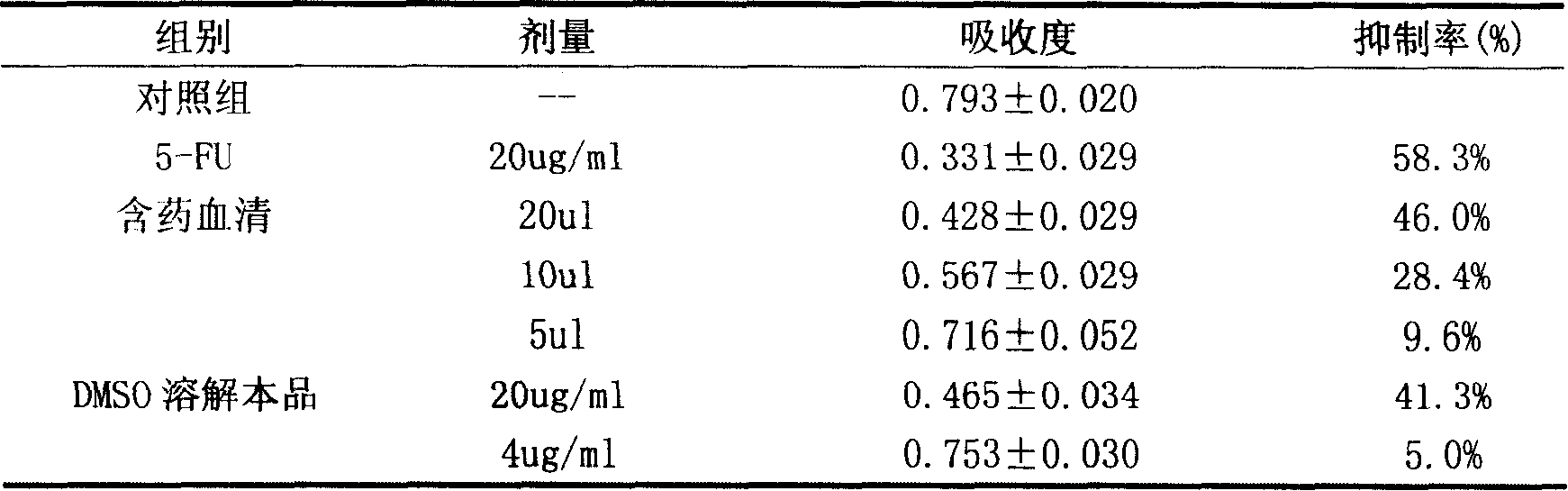
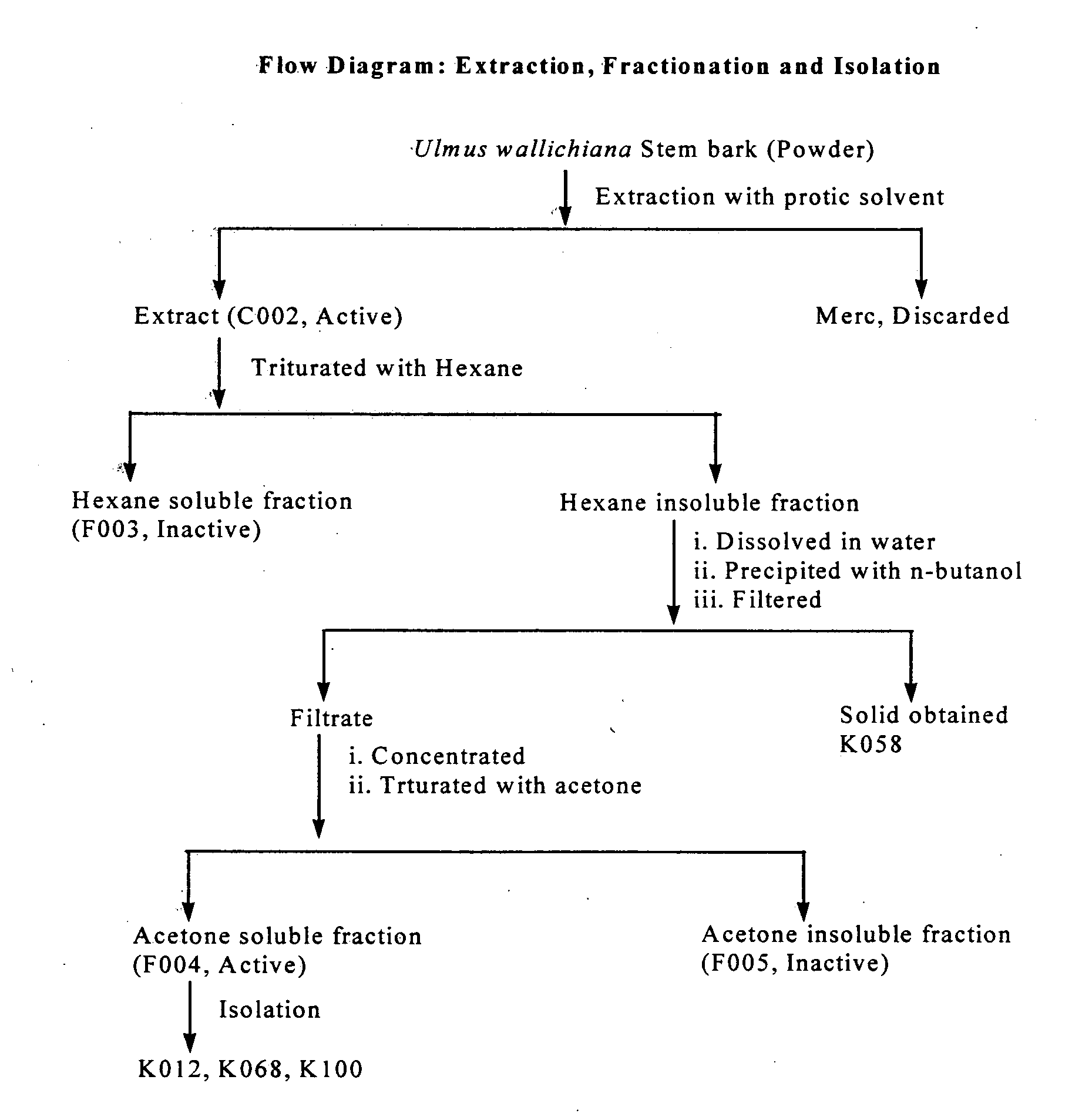
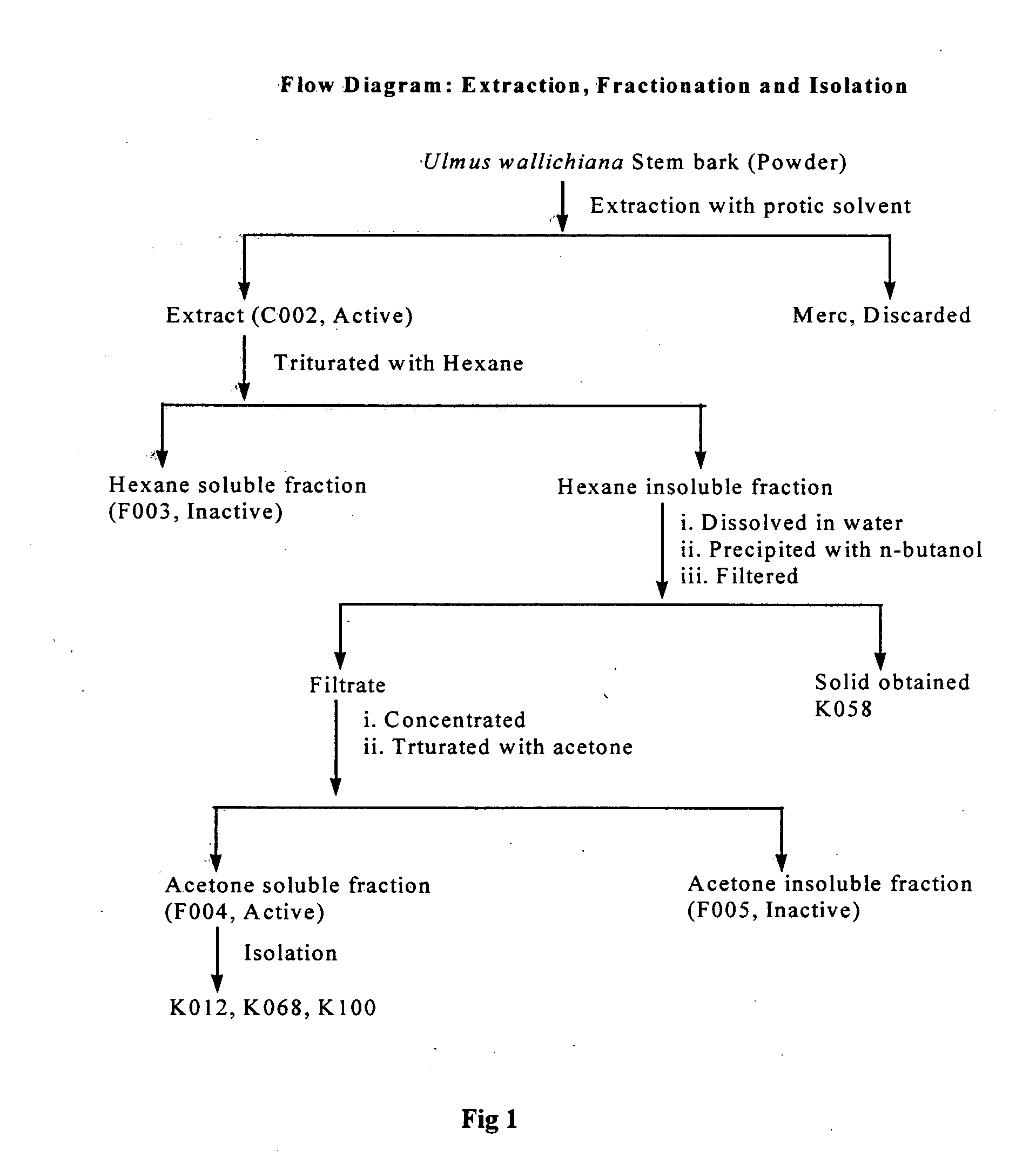
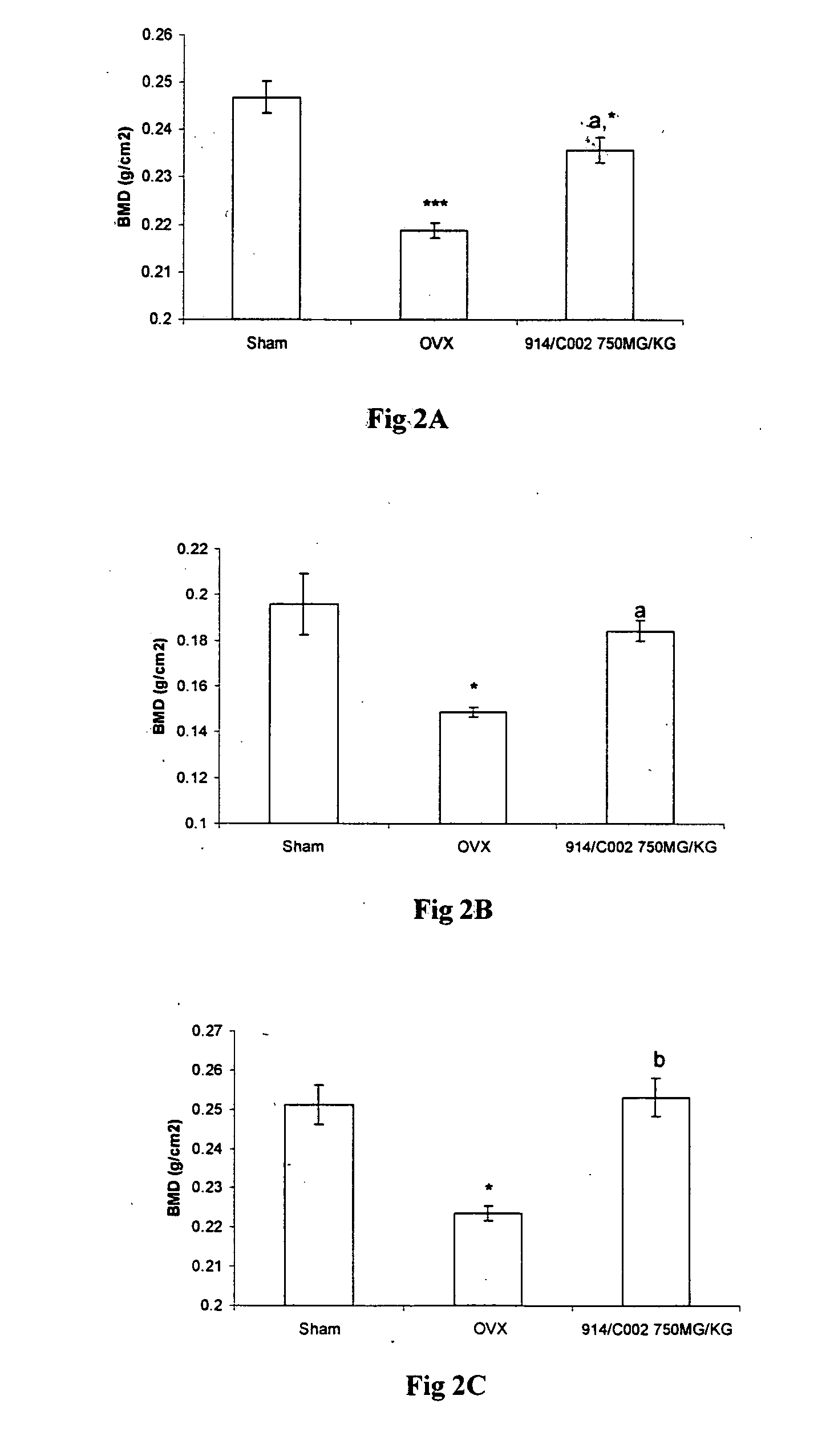
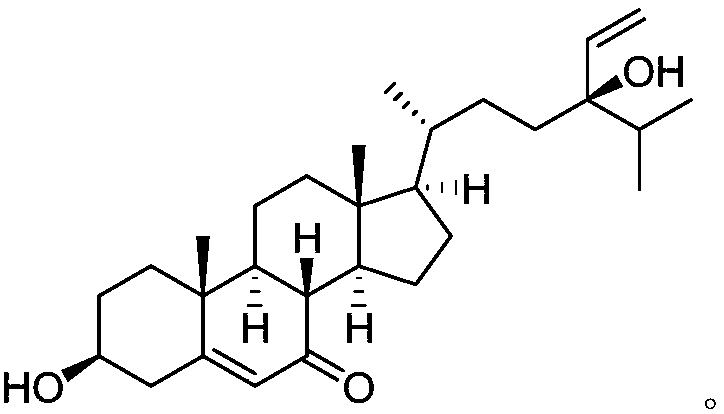
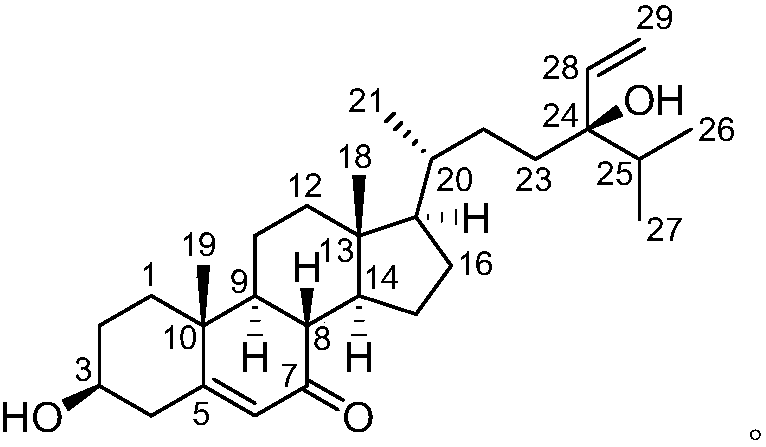
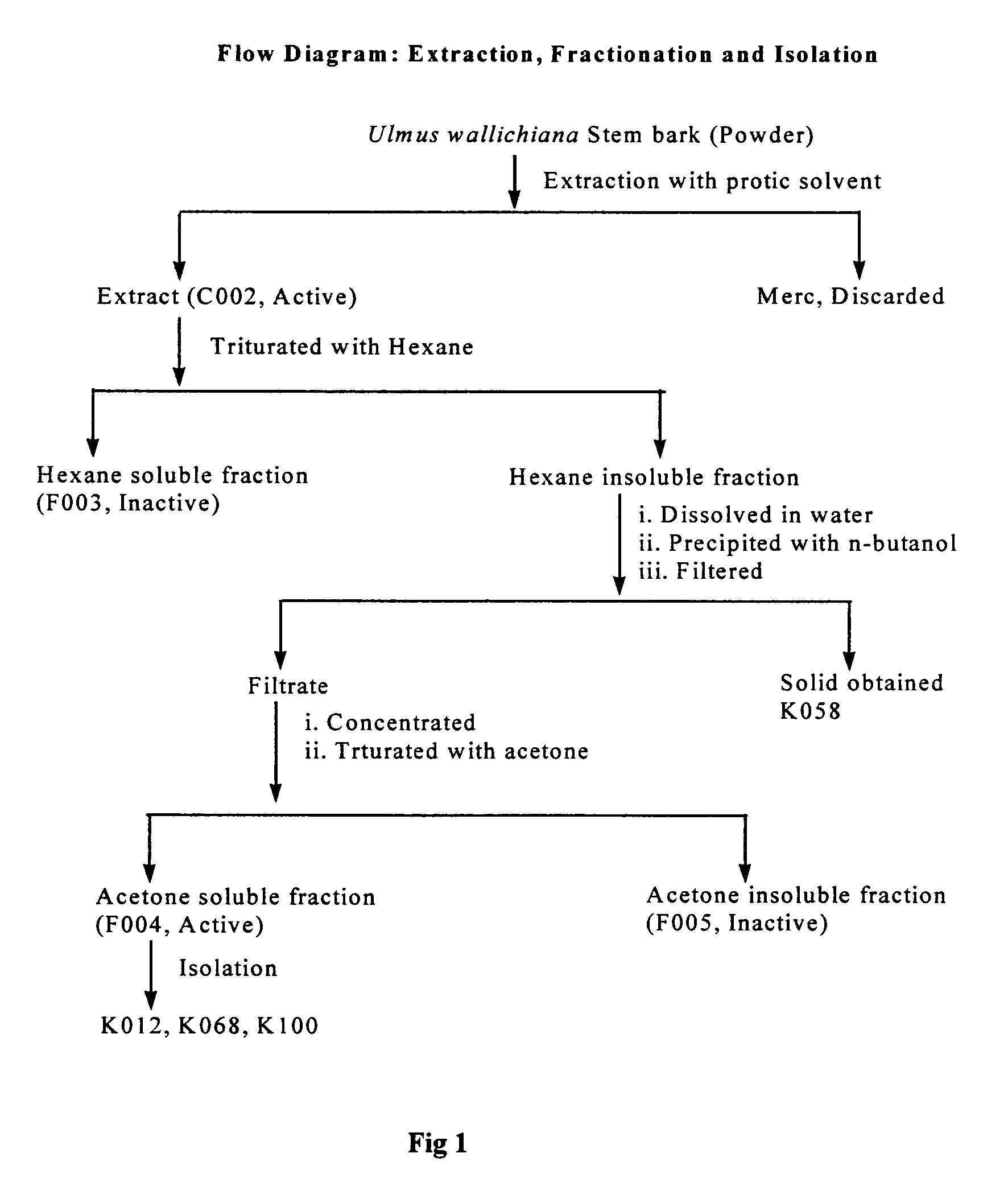
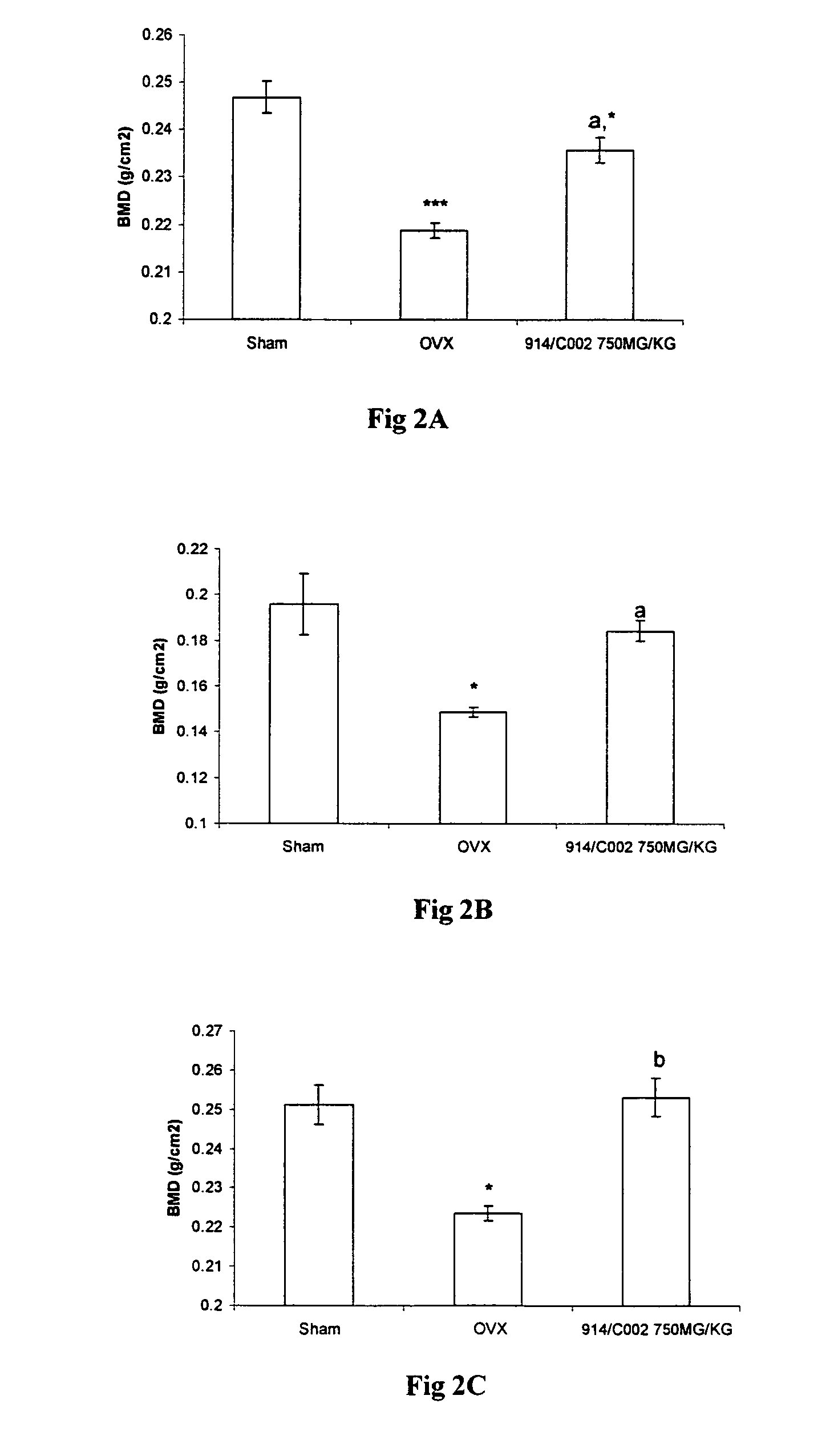
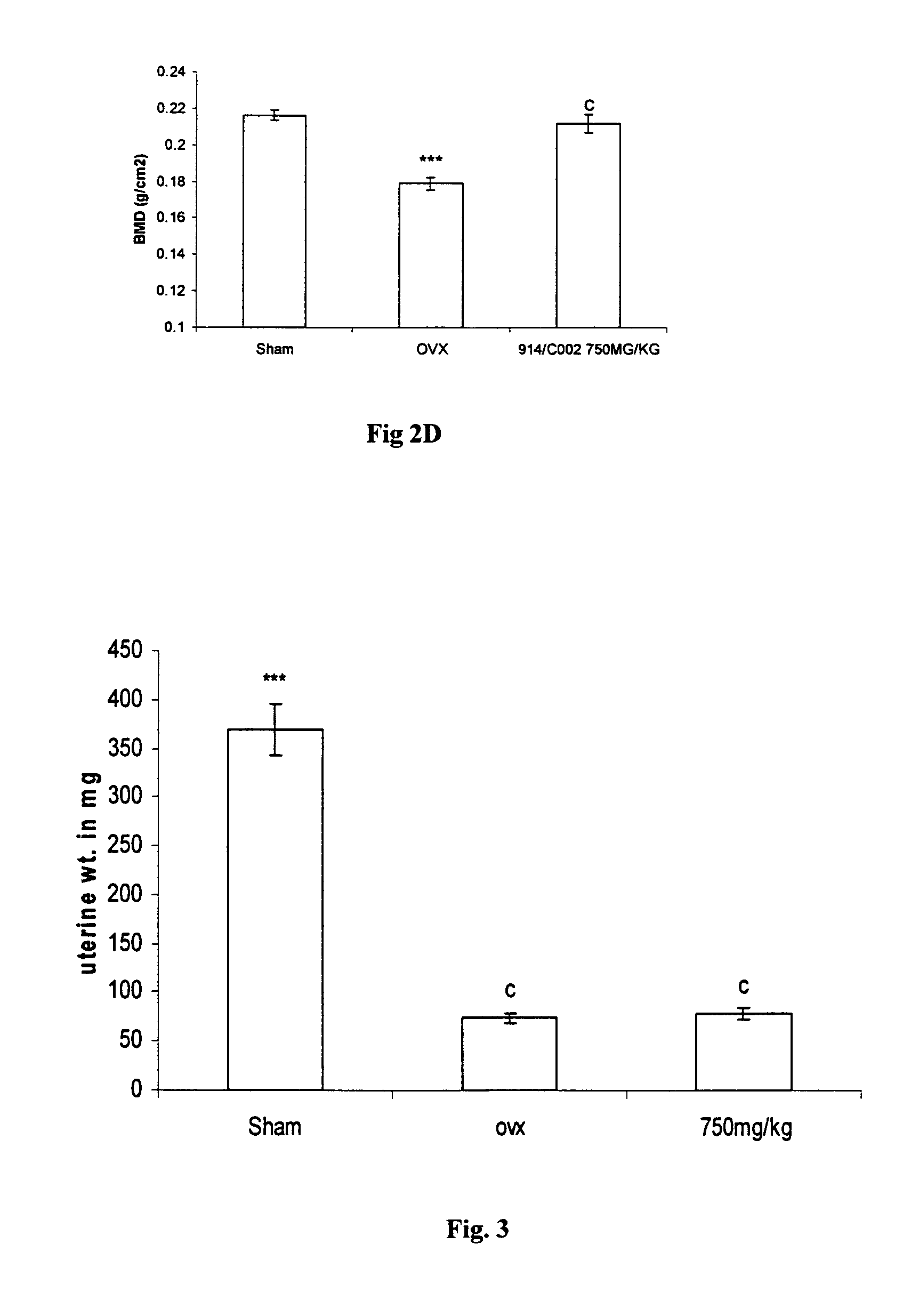
Novel flavonol compounds, a bioactive extract/fraction from ulmus wallichiana and its compounds for prevention for treatment of osteo-health related disorders
InactiveUS20110112042A1Reduces Ovx-induced bone lossBiocideSaccharide with heterocyclic radicalsDiseaseDouble bond
The present invention provides a flavonol compound and a bioactive extract / fraction from Ulmus wallichiana useful for the management or prevention or treatment of bone disorders. Said extract / fraction comprising marker compounds of general formula 2, K058: R1═R2═0H K012: R1═R2═OH, 2,3 double bond K068: R1═R2═H KIOOi R1═OH, R2═H mula 2 Wherein the marker compounds K012, K058, K068, K100 ranges 6.7-12%, 1.7-4.5%, 0.6-1.2%, 1.7-4.5% respectively in alcoholic extract and a process of extraction thereof. According to another aspect of the invention provides a pharmaceutical composition comprising the said compound. The present invention further provides a method of treating bone disorders by administering the pharmaceutical composition by oral, intravenous, subcutaneous, intra-peritoneal or intramuscular route.
Owner:COUNCIL OF SCI & IND RES
Steroidal natural drug with antitumor effect as well as preparation method and application of steroidal natural drug
The invention relates to the technical field of medicines and in particular relates to a novel natural drug which is extracted and separated from Chinese Dictyopteris undulate Holmes and has an antitumor effect. The drug is a steroidal compound dictyopterisin G. An in-vitro antitumor activity test shows that the IC50 values of the compound to a human acute promyelocytic leukemic cell (HL-60) and ahuman lung cancer cell (A-549) are respectively 2.70mu M and 2.01mu M, and therefore, the compound can be used for preparing an antitumor drug, can be prepared into an antitumor drug composition together with other drugs and can be prepared into dosage forms such as an injection, a tablet, a pill, a capsule, a solution, a suspension agent and an emulsion together with pharmaceutically acceptableauxiliary materials; and the administration route can be oral administration, intravenous administration, intramuscular administration or transdermal administration.
Owner:NANCHANG UNIV
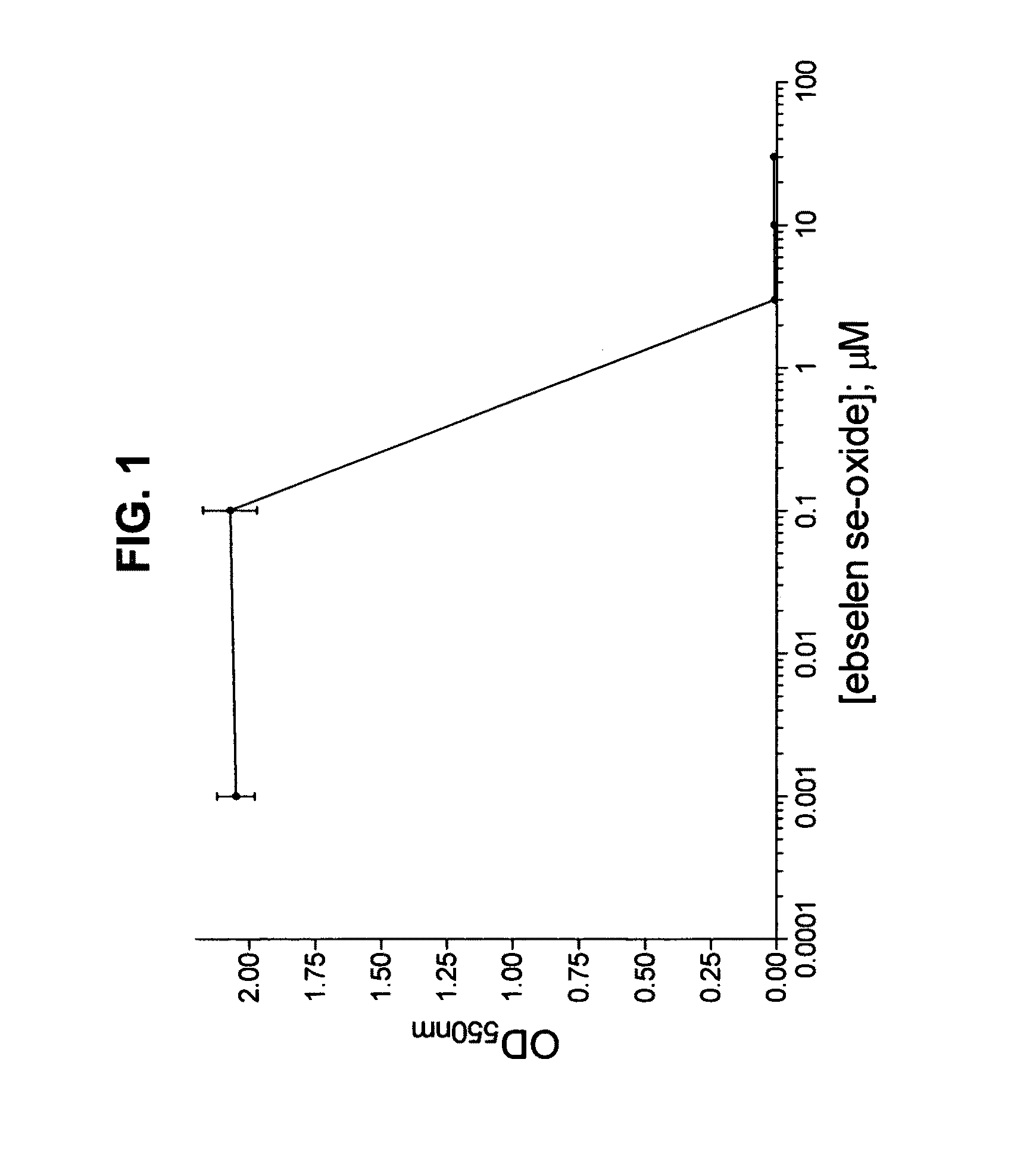
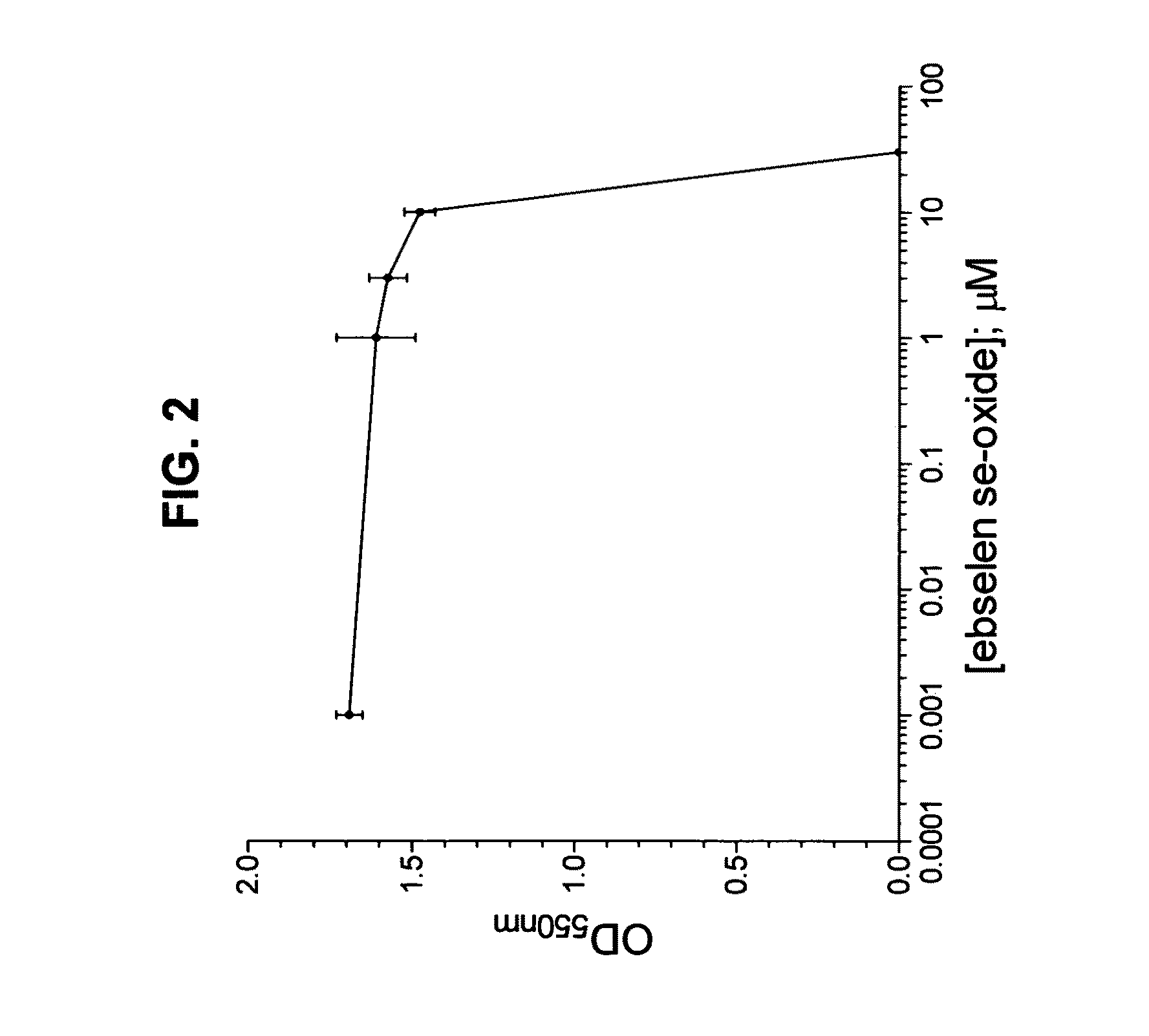
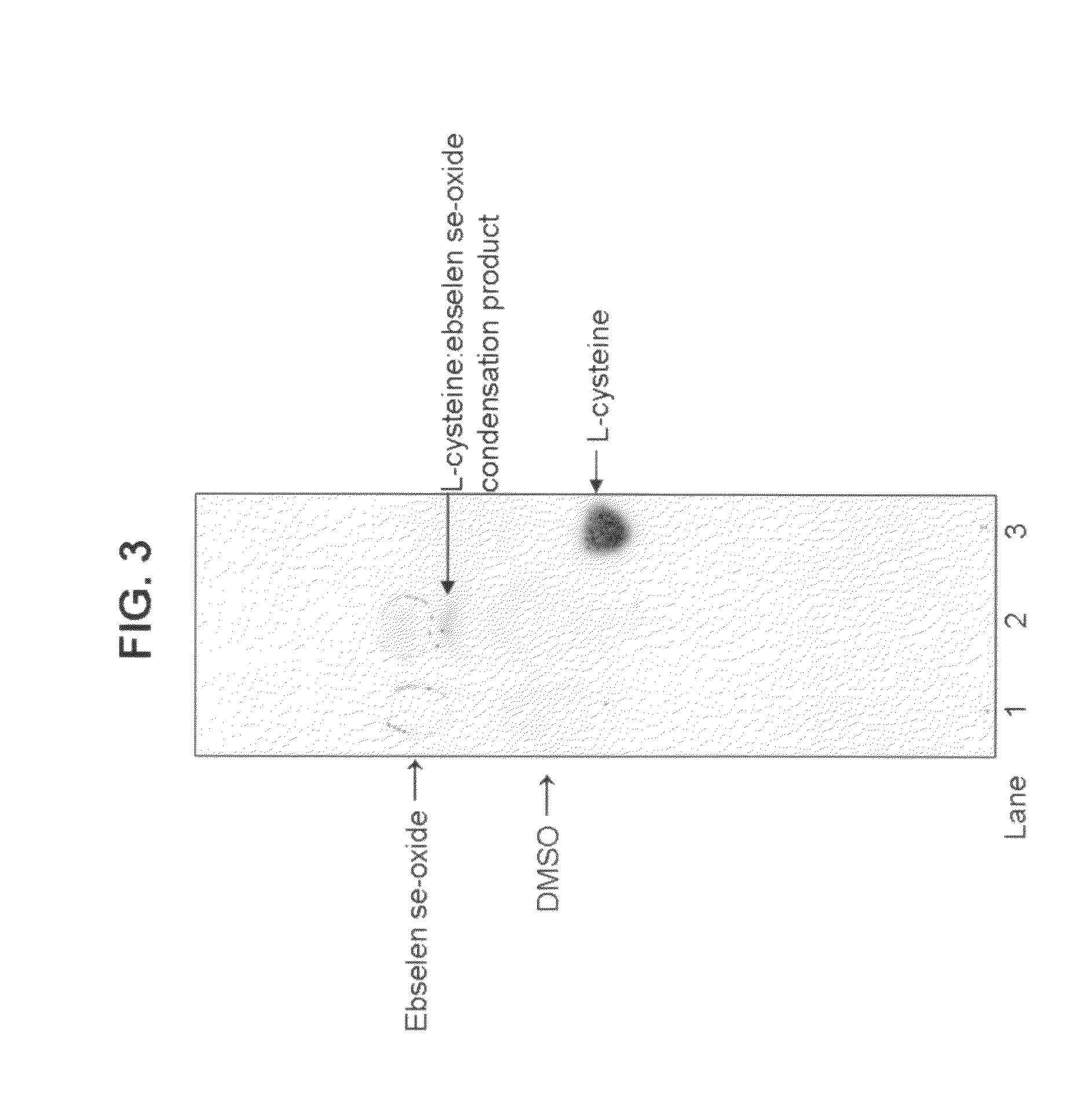
Process for the treatment of bacterial infections using 2-phenyl-1,2-benzisoselenazol-3(2H)-one 1-oxide
Owner:BILLACK BLASE CHRISTOPHER +1
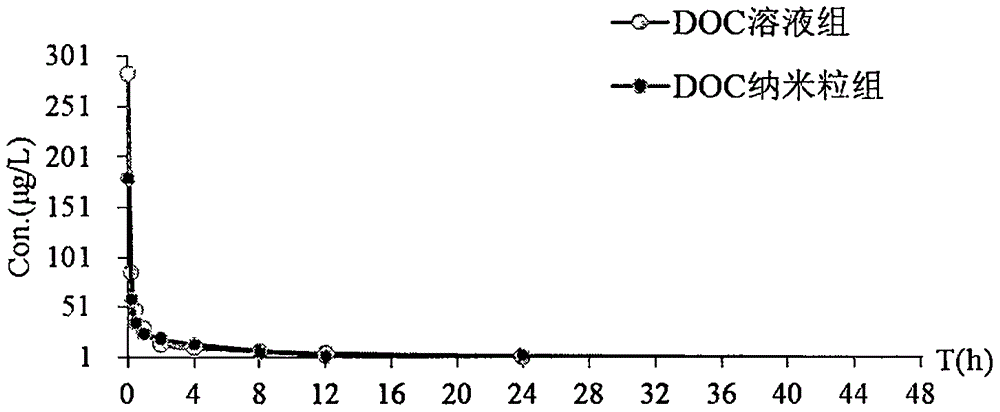
Palmitoyl ascorbic acid ester and docetaxel composite nanoparticles
InactiveCN105997982AHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsPowder deliveryDocetaxel-PNPDocetaxel
The invention relates to a composite nanoparticle preparation for injection administration for treatment of tumors, in particular to compound palmitoyl ascorbic acid ester and docetaxel composite nanoparticles and a preparation method thereof. Through combination of palmitoyl ascorbic acid ester and docetaxel, it is found through in-vitro and in-vivo experiments that the composite nanoparticles can remarkably enhance the anti-tumor efficacy of docetaxel, and docetaxel can achieve the efficacy at an extremely-low dose. Through intravenous administration, artery administration, intramuscular administration, subcutaneous administration, intraperitoneal administration and other administration modes, especially interventional administration such as intravenous injection and intratumoral injection, the effectiveness and safety of clinical application of docetaxel are improved. The adopted preparation method is simple in process and low in cost. The prepared nanoparticles are uniform in particle size, high in encapsulation rate and good in drug release property. Compared with palmitoyl ascorbic acid ester nanoparticles or docetaxel single-recipe nanoparticles, the effects of inhibiting tumor cell growth in vitro and resisting tumor proliferation in vivo of the compound are enhanced remarkably.
Owner:陈西敬
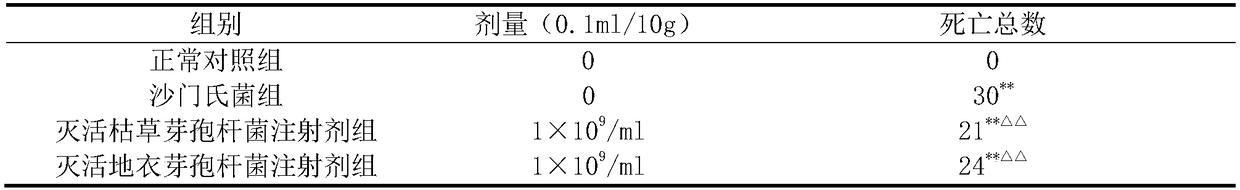
Inactivated probiotic bacillus injection
The invention relates to an antibacterial and anti-virus injection and belongs to the field of biomedicines. A main component of the medicine is inactivated probiotic bacillus which is obtained by inactivating living probiotic bacillus. Inactivated probiotic bacillus intramuscular administration has antibacterial and anti-virus effects, can be used for prevention, treatment or adjuvant treatment of various bacterial diseases and virus diseases of human or animals and has a wide application prospect.
Owner:SHANDONG AGRICULTURAL UNIVERSITY +1
A compound DICTYOPTERISINF and applications thereof in preparation of anti-tumor medicines
The invention relates to the technical field of medicines, and relates to a novel natural medicine having anti-tumor functions and extracted and separated from dictyopteris undulata Holmes in China. The medicine is a steroid compound that is dictyopterisin F. In-vitro anti-tumor activity tests prove that IC50 values of the compound for human acute promyelocytic leukemia cells (HL-60) and human lung cancer cells (A-549) are 2.45 [mu]M and 2.85 [mu]M respectively, and therefore the compound can be used for preparing anti-tumor medicines, can be prepared into anti-tumor medicine compositions withother medicines, and can be prepared into injections, tablets, pills, capsules, solutions, suspensions, emulsions, and other dosage forms with pharmaceutically acceptable auxiliary materials, and administration routes of the compound can be oral administration, intravenous administration, intramuscular administration or percutaneous administration.
Owner:HUNAN STEROL PHARM TECH CO LTD
Methods for treating plague
ActiveUS20170296644A1Bacterial antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsPolynucleotidePneumonic plague
Provided herein are methods for using compositions that include a fusion protein having a YscF protein domain, a mature F1 protein domain, and a LcrV protein domain. In one embodiment the composition is used to confer immunity to plague, such as pneumonic plague, caused by Yersinia pestis. In one embodiment, the composition is administered to a mucosal surface, such as by an intranasal route. In one embodiment, the administration to a mucosal surface includes a vector that has a polynucleotide encoding a fusion protein, where the fusion protein includes a YscF protein domain, a mature F1 protein domain, and a LcrV protein domain. The administration is followed by a second administration by a different route, such as an intramuscular route. The second administration includes a fusion protein having the same three domains, and in one embodiment the fusion protein is the same one administered to a mucosal surface.
Owner:WESTPORT BIO LLC +1
Pharmaceutical Compositions Comprising Paracetamol and Process for Preparing The Same
InactiveUS20170165210A1Organic active ingredientsPharmaceutical delivery mechanismHigh concentrationAntifungal
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
Pharmaceutical Compositions Comprising Paracetamol and Process for Preparing The Same
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
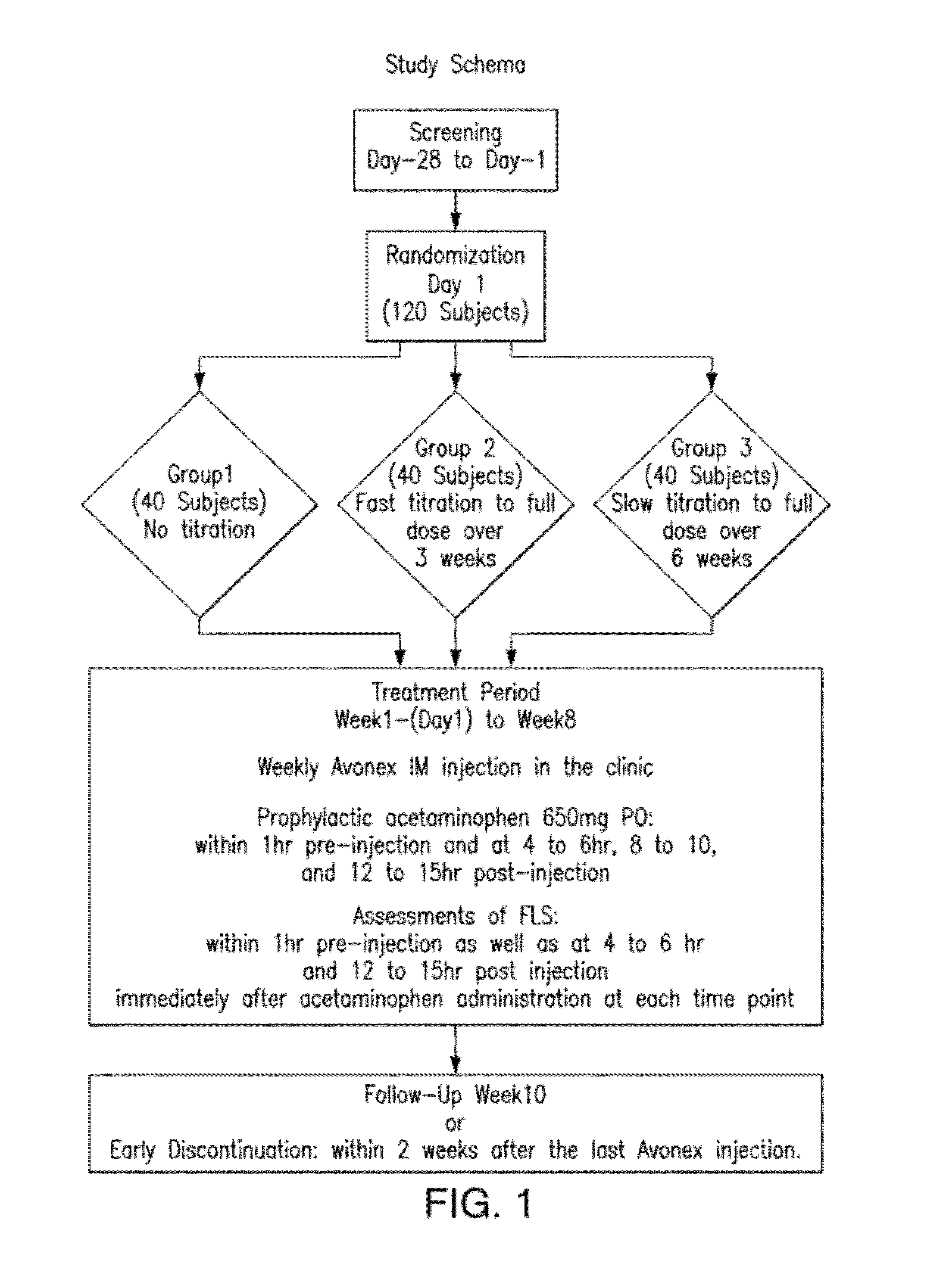
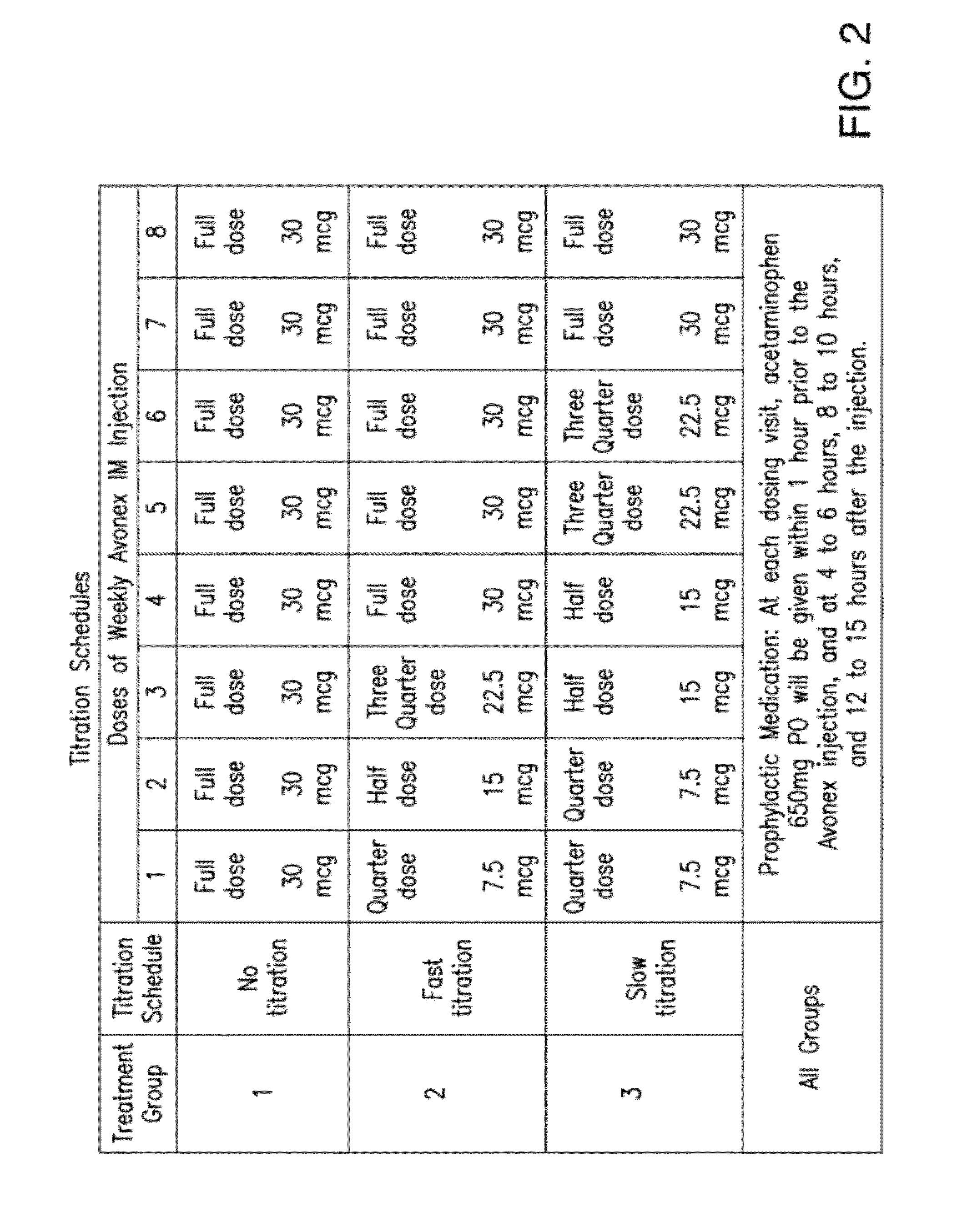
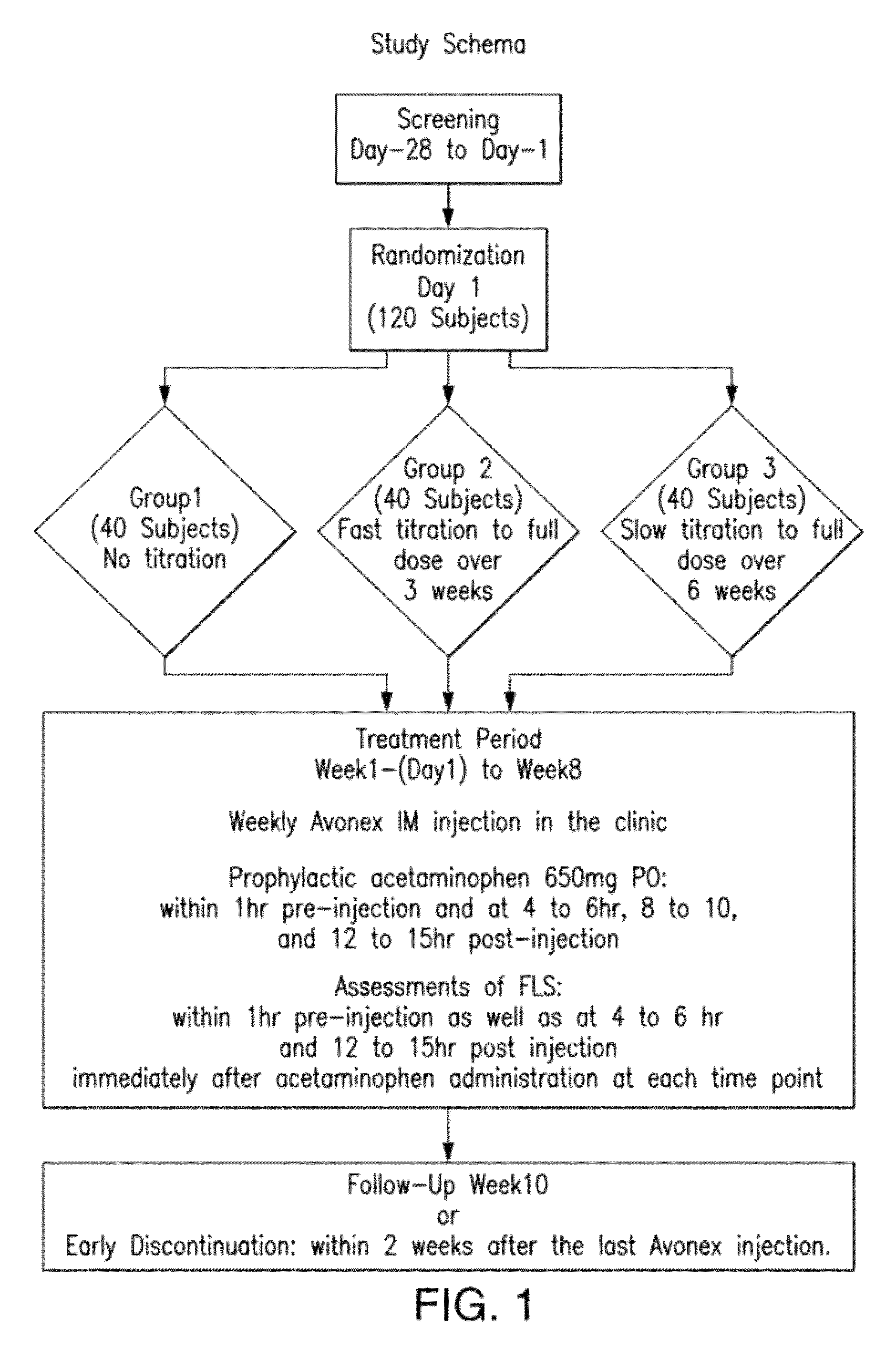
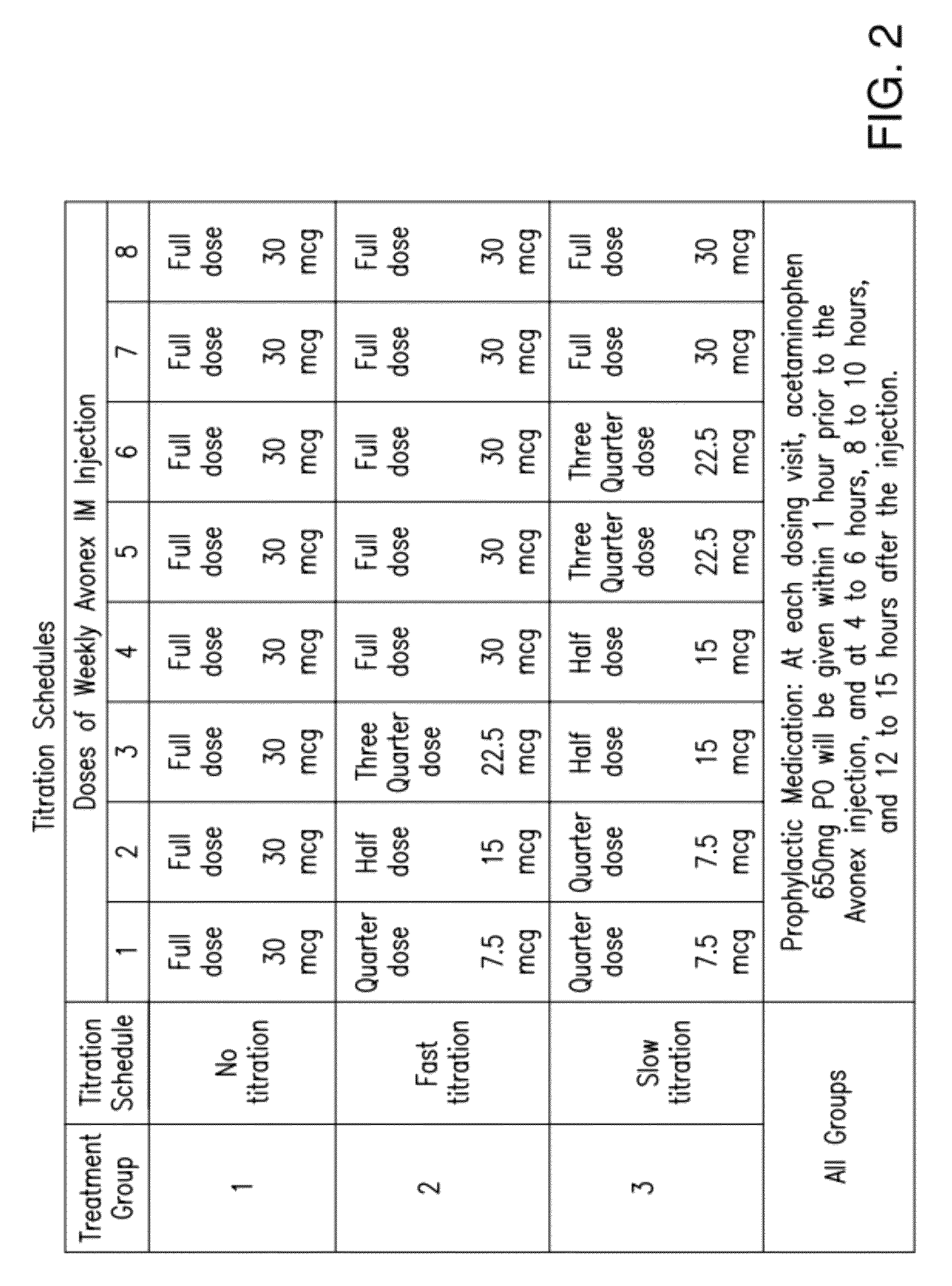
Method for reducing flu-like symptoms associated with intramuscular administration of interferon using a fast titration escalating dosing regimen
ActiveUS20120237479A1Reduce appearance problemsRelieve symptomsNervous disorderJet injection syringesDosing regimenRegimen
The present invention provides a method for treating multiple sclerosis (MS), and for reducing flu-like symptoms associated with administration of an interferon to a patient with MS. The method involves intramuscularly administering the interferon to the MS patient according to an escalating dosing regimen in weeks 1 to 3, and a full therapeutically effective dose of interferon in week 4. In one embodiment of the invention, the escalating dosing regimen comprises administering one quarter of the therapeutically effective dose in week 1, half of the therapeutically effective dose in week 2, and three-quarters of the therapeutically effective dose in week 3. Also provided are titration packages for enabling compliance with a regimen of changing dosage of an interferon over a period of time.
Owner:BIOGEN MA INC
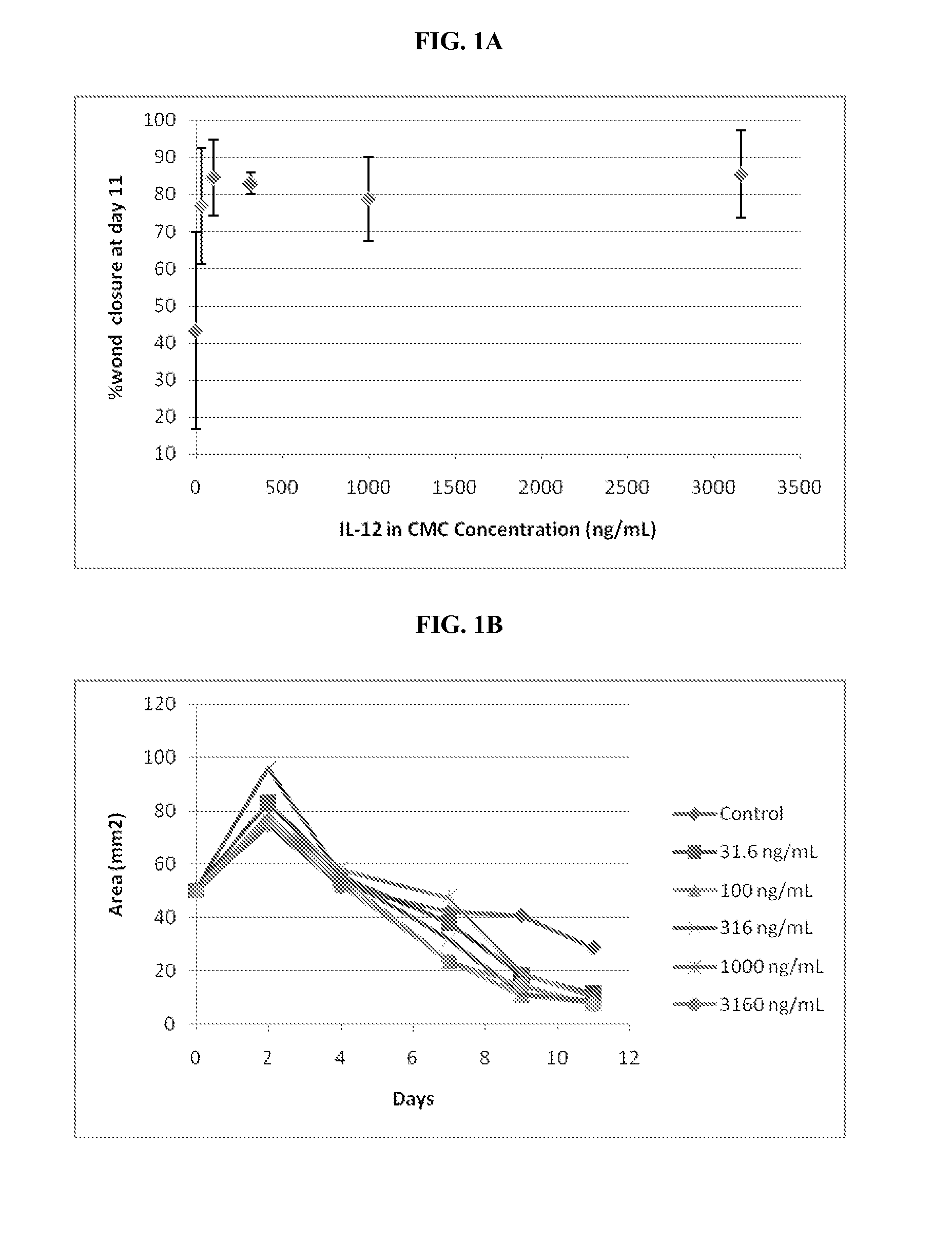
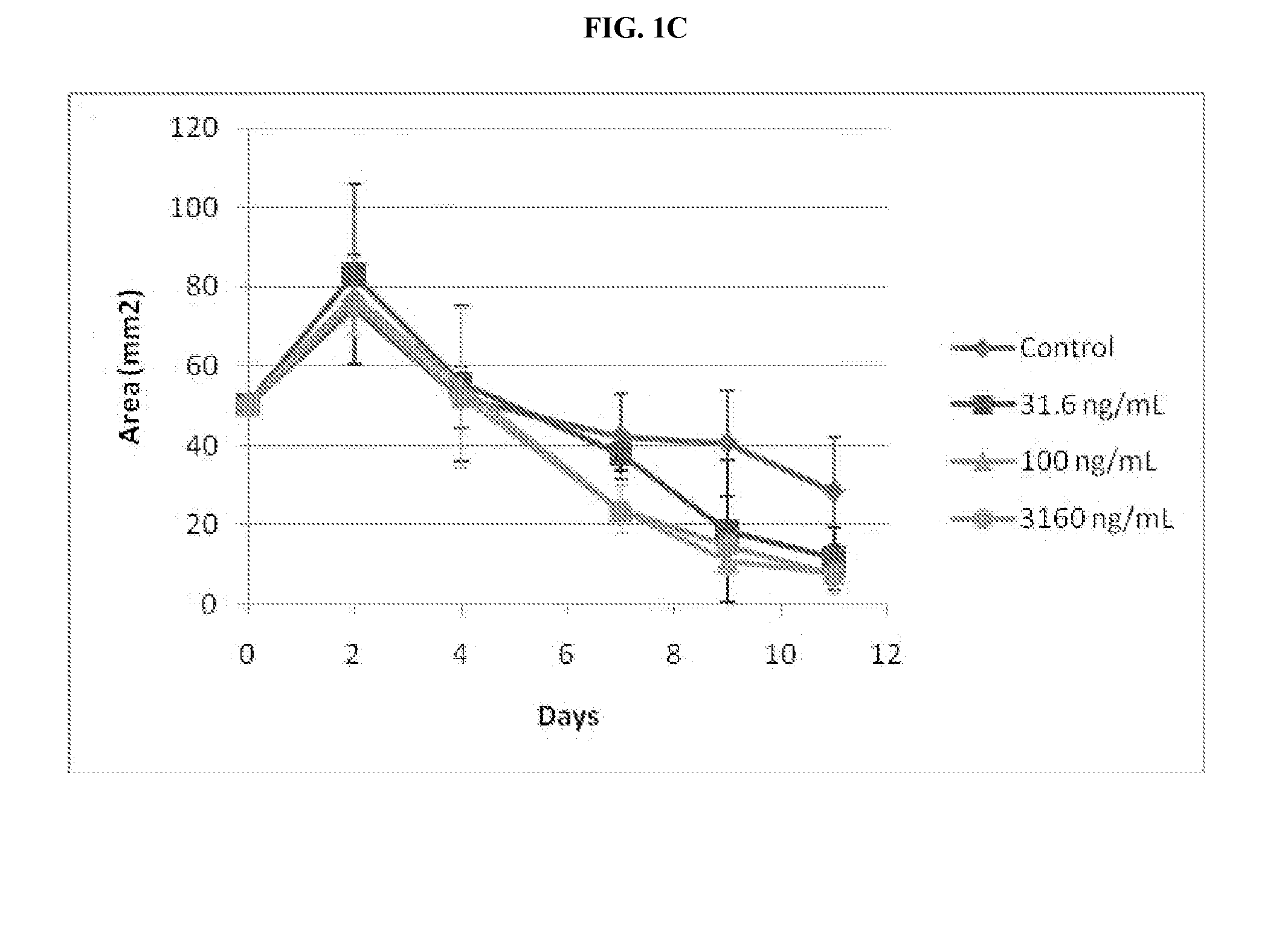
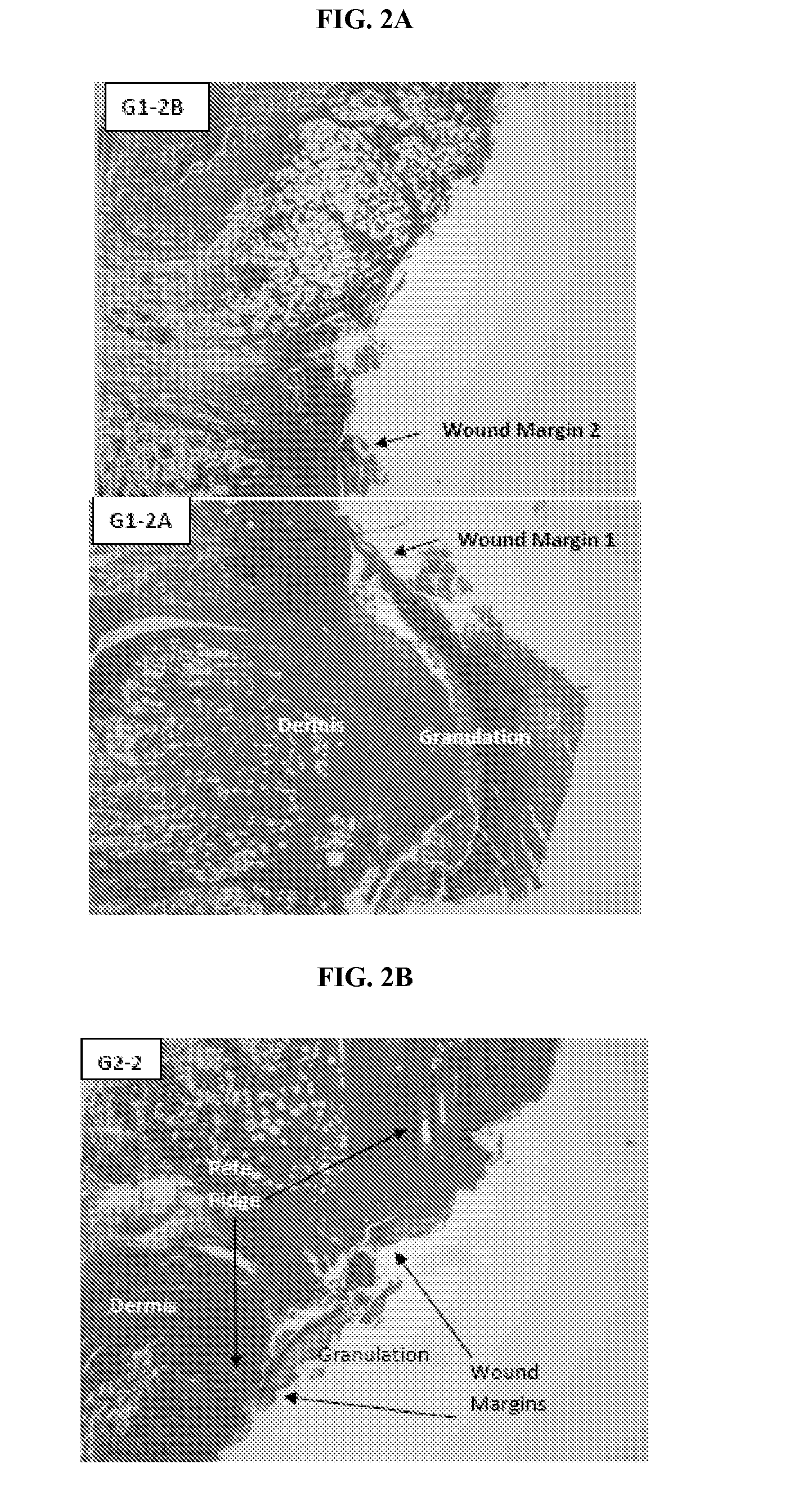
Mitigation of cutaneous injury with il-12
ActiveUS20140205561A1Peptide/protein ingredientsPharmaceutical delivery mechanismCutaneous woundSurgery
The present application relates to treatment of cutaneous wounds using IL-12. The methods of the invention result in improved wound closure. The methods comprise treating cutaneous wounds using topical, subcutaneous, and / or intramuscular administration of IL-12.
Owner:NEUMEDICINES INC
Method for reducing flu-like symptoms associated with intramuscular administration of interferon using a fast titration escalating dosing regimen
ActiveUS9198955B2Reduce appearance problemsRelieve symptomsNervous disorderJet injection syringesDosing regimenRegimen
The present invention provides a method for treating multiple sclerosis (MS), and for reducing flu-like symptoms associated with administration of an interferon to a patient with MS. The method involves intramuscularly administering the interferon to the MS patient according to an escalating dosing regimen in weeks 1 to 3, and a full therapeutically effective dose of interferon in week 4. In one embodiment of the invention, the escalating dosing regimen comprises administering one quarter of the therapeutically effective dose in week 1, half of the therapeutically effective dose in week 2, and three-quarters of the therapeutically effective dose in week 3. Also provided are titration packages for enabling compliance with a regimen of changing dosage of an interferon over a period of time.
Owner:BIOGEN MA INC
Methods for treating plague
ActiveUS10076562B2Bacterial antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsNucleotidePolynucleotide
Provided herein are methods for using compositions that include a fusion protein having a YscF protein domain, a mature F1 protein domain, and a LcrV protein domain. In one embodiment the composition is used to confer immunity to plague, such as pneumonic plague, caused by Yersinia pestis. In one embodiment, the composition is administered to a mucosal surface, such as by an intranasal route. In one embodiment, the administration to a mucosal surface includes a vector that has a polynucleotide encoding a fusion protein, where the fusion protein includes a YscF protein domain, a mature F1 protein domain, and a LcrV protein domain. The administration is followed by a second administration by a different route, such as an intramuscular route. The second administration includes a fusion protein having the same three domains, and in one embodiment the fusion protein is the same one administered to a mucosal surface.
Owner:WESTPORT BIO LLC +1
Flavonol compounds, a bioactive extract/fraction from Ulmus wallichiana and its compounds for prevention for treatment of osteo-health related disorders
The present invention provides a flavonol compound and a bioactive extract / fraction from Ulmus wallichiana useful for the management or prevention or treatment of bone disorders. Said extract / fraction comprising marker compounds of general formula 2, K058: R1═R2═OH K012: R1═R2═OH, 2,3 double bond K068: R1═R2═H KIOOi R1═OH, R2═H mula 2 Wherein the marker compounds K012, K058, K068, K1OO ranges 6.7-12%, 1.7-4.5%, 0.6-1.2%, 1.7-4.5% respectively in alcoholic extract and a process of extraction thereof. According to another aspect of the invention provides a pharmaceutical composition comprising the said compound. The present invention further provides a method of treating bone disorders by administering the pharmaceutical composition by oral, intravenous, subcutaneous, intra-peritoneal or intramuscular route.
Owner:COUNCIL OF SCI & IND RES
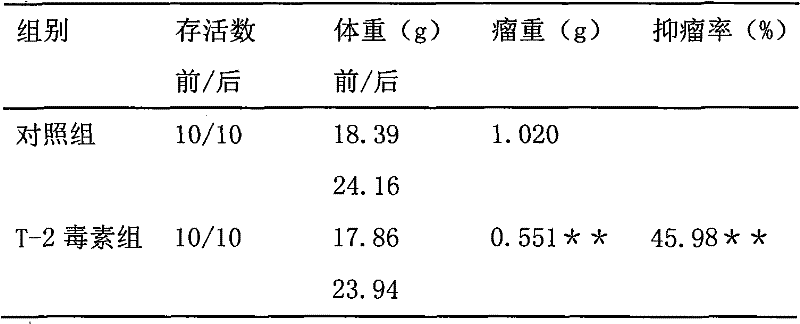
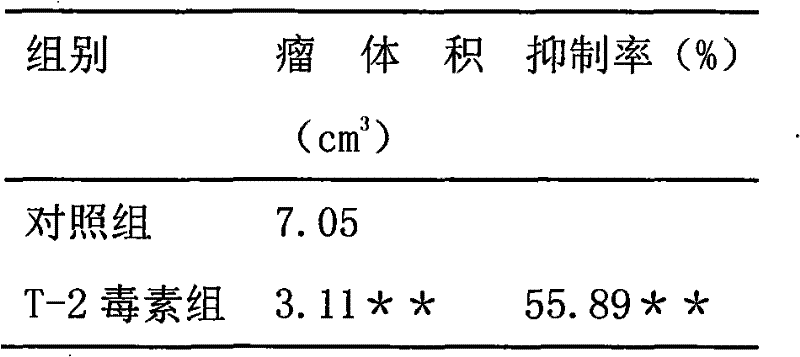
Application of t-2 toxin in preparation of medicine for treating brain tumors
ActiveCN101984962BDegree of reductionEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsSide effectWilms' tumor
The invention discloses a new application of T-2 toxin, that is, the application of T-2 toxin in the preparation of medicines for treating brain tumors. The effective therapeutic dose is 0.1-20 mg / Kg body weight, preferably 0.5-8 mg / Kg body weight The administration may be implantation administration, oral administration, intravenous injection administration, subcutaneous injection administration, intramuscular injection administration, preferably intratumoral injection. The inventors of the present application established various solid tumor models, and then injected T-2 toxin into the tumor or co-cultured with cells to investigate the inhibitory effect of T-2 toxin on tumor or tumor cells. Experiments have shown that intratumoral injection has a definite curative effect on tumors, especially solid tumors that are difficult to remove, and intratumoral injection can further reduce the scope and degree of side effects of T-2 toxin, reduce its side effects and improve the efficacy of T-2 toxin. Inhibition and killing of tumor tissue.
Owner:SHENZHEN ICARBONX INTELLIGENT PEPTIDE PHARM TECH CO LTD
Externally used traditional Chinese medicine for relieving pain and promoting healing after hepatobiliary surgery
InactiveCN106266290AAnalgesic effect is effectiveDefinite curative effectAnthropod material medical ingredientsAntipyreticSide effectBengal kino
The invention discloses an externally used traditional Chinese medicine for relieving pain and promoting healing after a hepatobiliary surgery. The externally used traditional Chinese medicine for relieving pain and promoting healing after the hepatobiliary surgery is prepared from the following raw materials in parts by weight: 10-20 parts of Herba Sedi, 5-15 parts of Caragana brevifolia Komar., 6-14 parts of Adeps melis, 10-20 parts of Caragana jubata (Pall.) Poir., 4-6 parts of Butea monosperma, 15-25 parts of Cortex Firmianae, 15-25 parts of Radix Ampelopsis, 1-5 parts of Radix Salviae Miltiorrhizae, 1-5 parts of Radix Angelicae Sinensis, 10-20 parts of Radix Arnebiae, 10-20 parts of Radix Glycyrrhizae and 1-3 parts of Radix seu Caulis Acanthi Ilicifolii. The externally used traditional Chinese medicine has effects of promoting blood circulation and dispelling blood stasis, and promoting the circulation of QI to relieve pain, directly acts on an operative wound when externally used, enters the grain of skin and the texture of the subcutaneous flesh from pores by applying the medicine on the outside of the skin, and has effects of inducing menstruation and dredging collaterals to remove blood stasis and promote circulation of qi; and the effect of relieving pain is definite, toxic and side effects are small, addiction does not exist, resources are abundant, stimulation of the medicine to the intestines, the stomach, the liver and the gall is reduced, local dressing can be realized, the medicine directly reaches the focus to promote the effect of reliving pain, pain of oral administration and intramuscular administration is avoided, and the development prospect is wide.
Owner:林丽
Application of compound Dictyopterisin I to preparation of antitumor drug
The invention relates to the technical field of medicines and in particular relates to a novel natural drug which is extracted and separated from Chinese Dictyopteris undulate Holmes and has an antitumor effect. The drug is a steroidal compound dictyopterisin I. An in-vitro antitumor activity test shows that the IC50 values of the compound to a human acute promyelocytic leukemic cell (HL-60) and ahuman lung cancer cell (A-549) are respectively 1.26mu M and 1.35mu M, and therefore, the compound can be used for preparing an antitumor drug, can be prepared into an antitumor drug composition together with other drugs and can be prepared into dosage forms such as an injection, a tablet, a pill, a capsule, a solution, a suspension agent and an emulsion together with pharmaceutically acceptableauxiliary materials; and the administration route can be oral administration, intravenous administration, intramuscular administration or transdermal administration.
Owner:上海耀大生物科技有限公司
Pharmaceutical compositions comprising paracetamol and process for preparing the same
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150 mg / ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent system comprising glycofurol, ethanol, polyethylene glycol, water. The viscosity of the said injectables is <28 cps. Further disclosed is the process for preparing the said injectables. The injectables can be administered by intramuscular route, intravenous route or as intravenous infusion after diluting in one of the routinely used intravenous fluids, infusion solutions of antibacterial, antifungal and amoebicidal drugs and along with anxiolytics (Midazolam injection) or narcotic analgesics (Fentanyl Citrate injection etc) as they remain stable, clear and transparent at least for 6 hours after dilution.
Owner:TROIKAA PHARMA
L-palmitoyl ascorbate/paclitaxel composition lipidosome
InactiveCN106166146AHigh encapsulation efficiencyGood stability and drug loadingOrganic active ingredientsAntineoplastic agentsCholesterolIn vivo
The invention discloses a composition lipidosome preparation used for parenteral administration tumor treatment. The composition lipidosome preparation comprises following ingredients: paclitaxel, L-palmitoyl ascorbate, a phospholipid substance, and a cholesterol analogue. According to a preparation method, L-palmitoyl ascorbate and paclitaxel are combined, the particle size of the obtained lipidosome is uniform, encapsulation efficiency is high, and drug releasing properties are excellent. It is found via in vivo and in vitro experiments that anti-tumor effect of paclitaxel is improved obviously; drug effect of paclitaxel can be achieved at a relatively low dosage; drug administration is realized via intravenous administration, artery administration, intramuscular administration, subcutaneous administration, intraperitoneal administration, and the like; and especially, clinical application availability and safety of paclitaxel are improved via interventional administration methods such as intravenous injection or intratumor injection.
Owner:陈西敬
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com