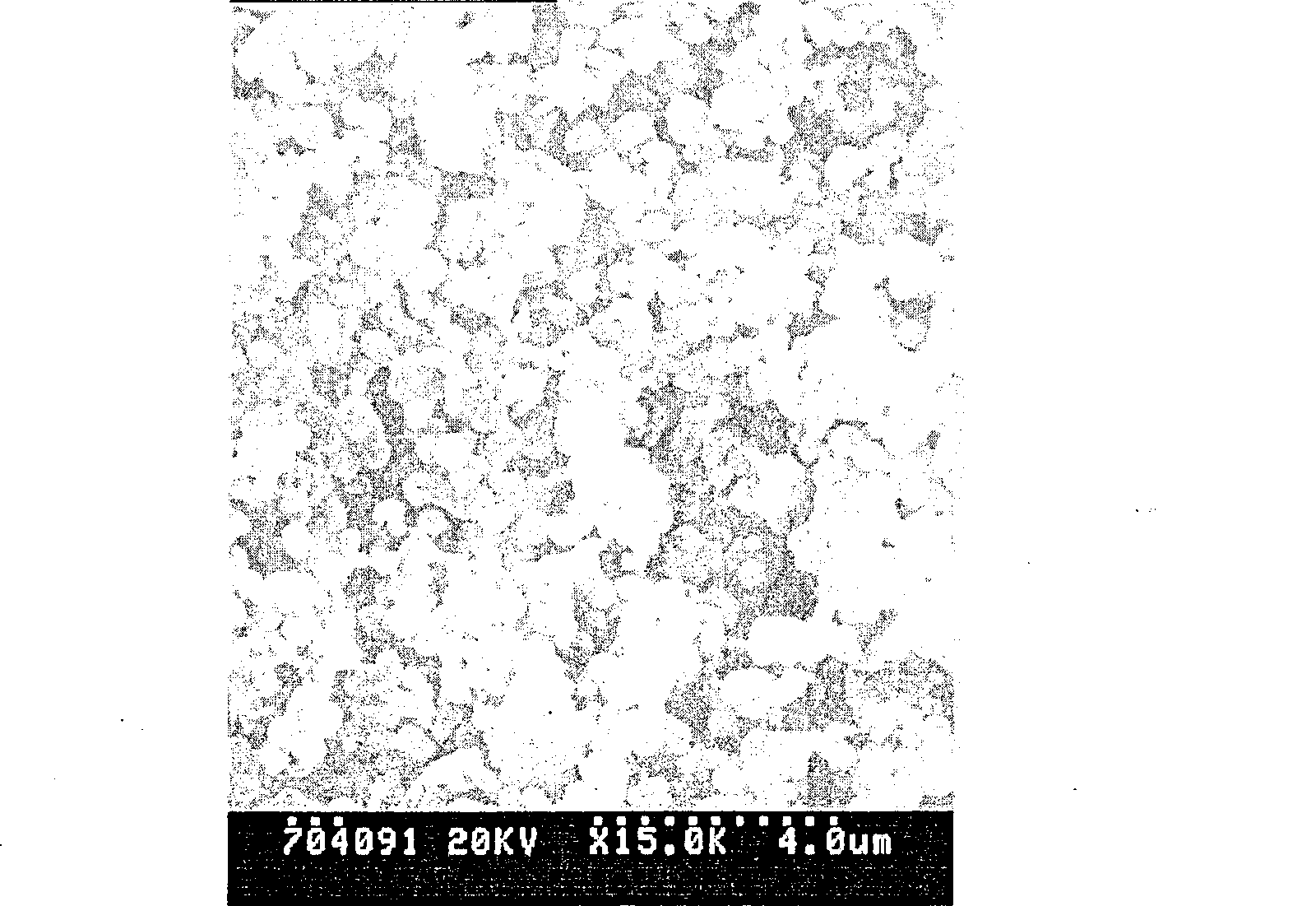
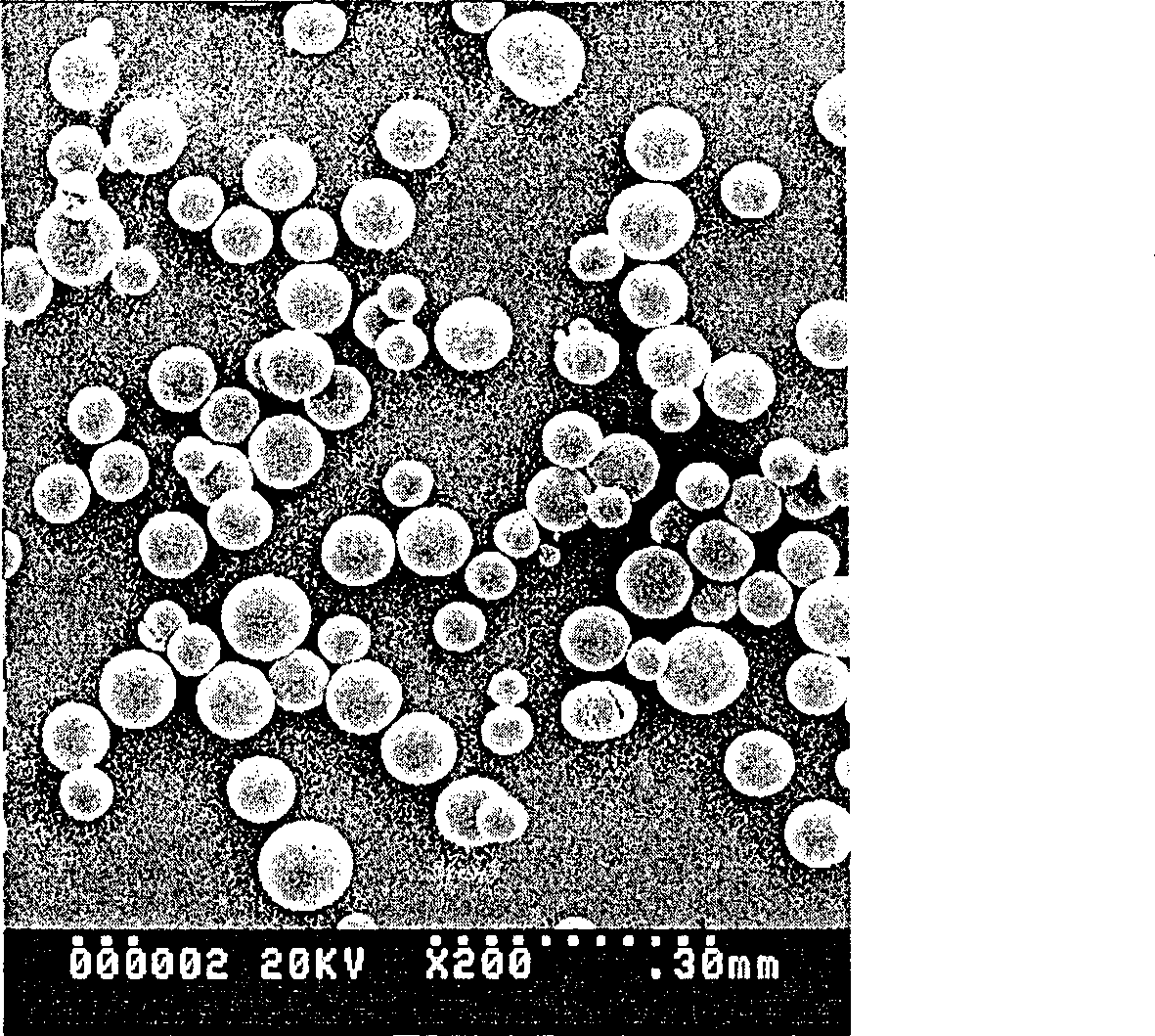
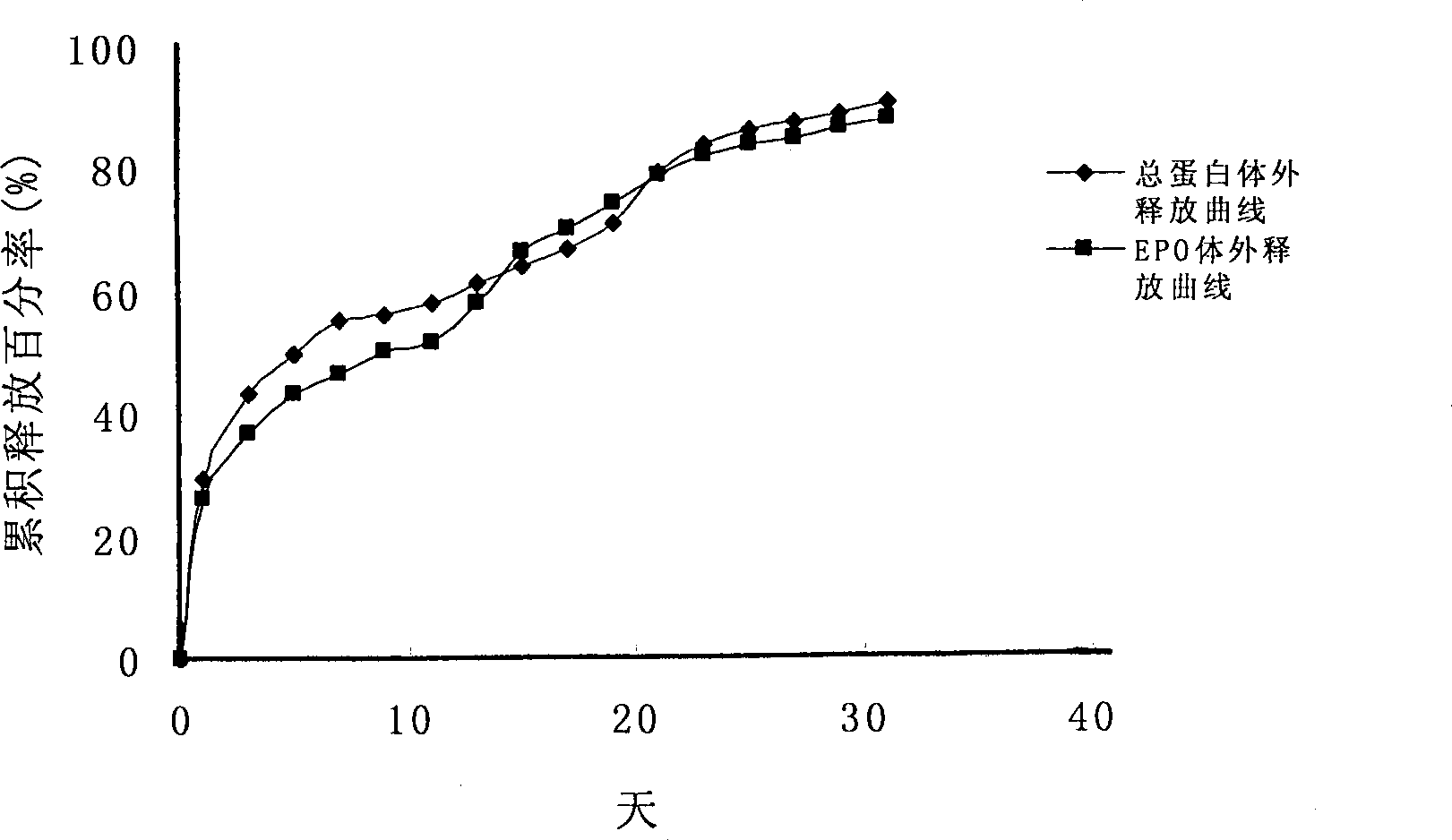
Sustained-release micro-spheres preparation containing recombined erythropoietin and preparation method and use thereof
A technology for erythropoietin and sustained-release microsphere preparation, which is applied in the field of lactic acid-glycolic acid block copolymer sustained-release microsphere preparation and its preparation, and can solve the problems of immune response, pure red aplastic anemia, drug loss of efficacy, etc. , to achieve good biocompatibility, high drug loading and encapsulation efficiency, and good protein stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Pre-freeze 10ml of a mixed solution of 0.5mg / ml recombinant human erythropoietin, 10mg / ml human serum albumin (HSA), and 45mg / ml polyethylene glycol at -80°C overnight, and routinely freeze-dry to obtain The obtained solid is continuously washed three times with dichloromethane to remove polyethylene glycol, and the mixed particles of recombinant human erythropoietin and human serum albumin are prepared. Emulsify the mixed particles of 40mg recombinant human erythropoietin and human serum albumin in 2ml of lactic acid-glycolic acid block copolymer dichloromethane solution with a concentration of 60mg / ml, under the condition that the stirring rate is 20000 rpm Emulsified for 60s to obtain S / O emulsion. Inject the emulsion into 250ml of 0.02M phosphate buffer (pH7.4) containing 2% polyvinyl alcohol with a micro sampler, stir at a speed of 600rpm for 1min to prepare the S / O / W emulsion, and add the emulsion to the emulsion Add 250ml 0.02M phosphate buffer solution to the s...
Embodiment 2
[0041] Pre-freeze 10ml of the mixed solution of 0.5mg / ml recombinant erythropoietin, 10mg / ml human serum albumin (HSA), and 30mg / ml polyethylene glycol at -80°C overnight, and routinely freeze-dry.
[0042] The obtained solid was washed continuously with dichloromethane for three times to remove polyethylene glycol, and mixed particles of recombinant erythropoietin and human serum albumin were prepared. The mixed particles of 40mg recombinant erythropoietin and human serum albumin were emulsified in 2ml of 120mg / ml lactic acid-glycolic acid block copolymer dichloromethane solution, and emulsified at a stirring rate of 20000 rpm 60s, get S / O emulsion. Inject the emulsion into 250ml of 0.02M phosphate buffer (pH is 7.4) containing 2% polyvinyl alcohol with a micro sampler, and stir at a speed of 600rpm for 1min to prepare the S / O / W emulsion. Add 250ml of 0.02M phosphate buffer solution to the solution, stir at 300rpm at room temperature for 6h, volatilize the organic solvent, c...
Embodiment 3
[0044] Pre-freeze 10ml of a mixed solution of 0.5mg / ml recombinant erythropoietin, 10mg / ml human serum albumin (HSA), and 45mg / ml polyethylene glycol at -80°C overnight, and routinely freeze-dry to obtain The solid matter was continuously washed with dichloromethane three times to remove polyethylene glycol, and the mixed microparticles of recombinant erythropoietin and human serum albumin were prepared. 40 mg of recombinant human erythropoietin and human serum albumin mixed particles were emulsified in 2 ml of 150 mg / ml lactic acid-glycolic acid block copolymer dichloromethane solution at a stirring rate of 20,000 rpm Emulsified for 60s to obtain S / O emulsion. Inject the emulsion into 250ml of 0.02M phosphate buffer (pH7.4) containing 2% PVA with a micro sampler, stir at a speed of 400rpm for 1min to prepare the S / O / W emulsion, and add Add 250ml of 0.02M phosphate buffer, stir at 300rpm at room temperature for 6h, volatilize the organic solvent, collect the microspheres by c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com