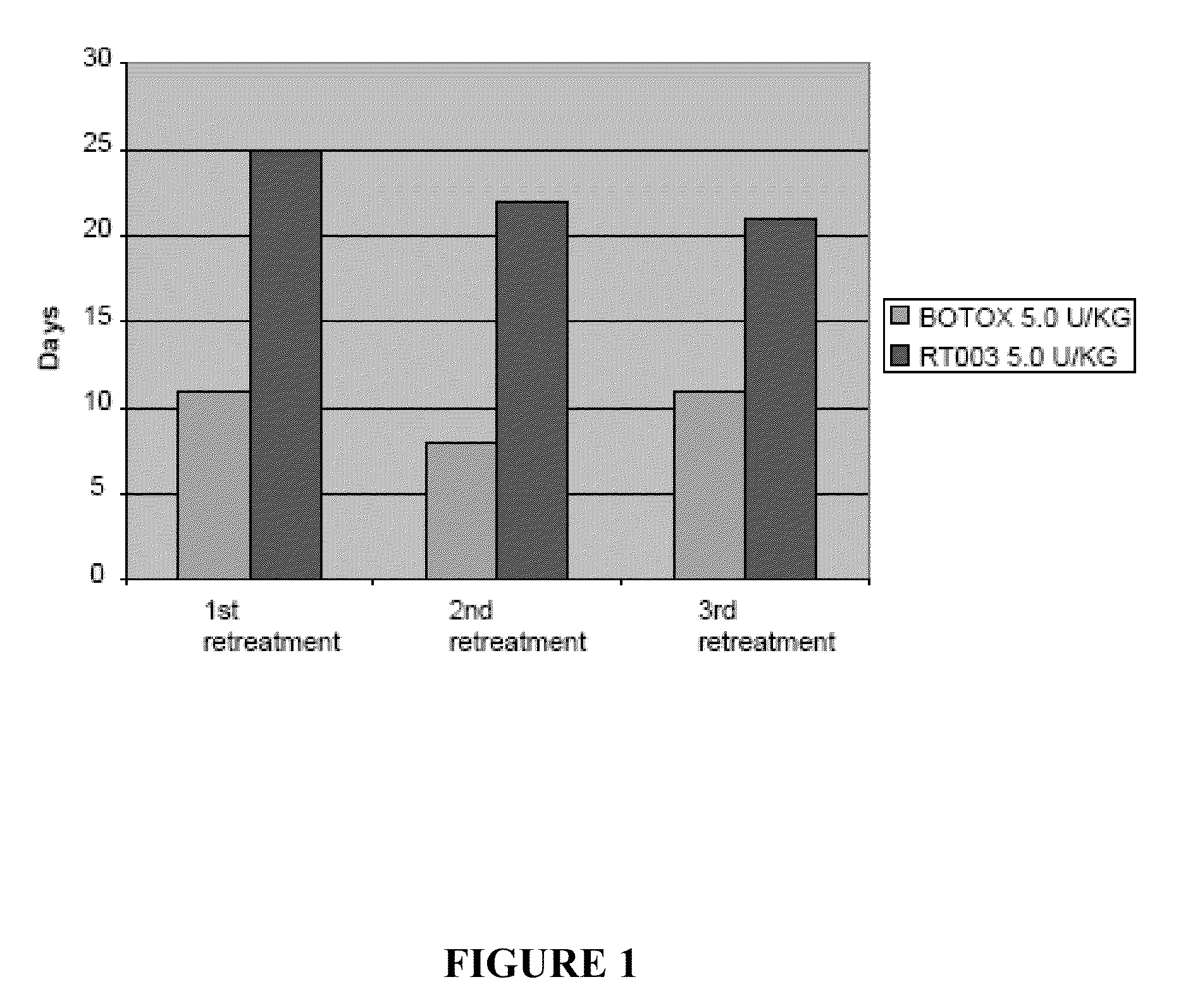
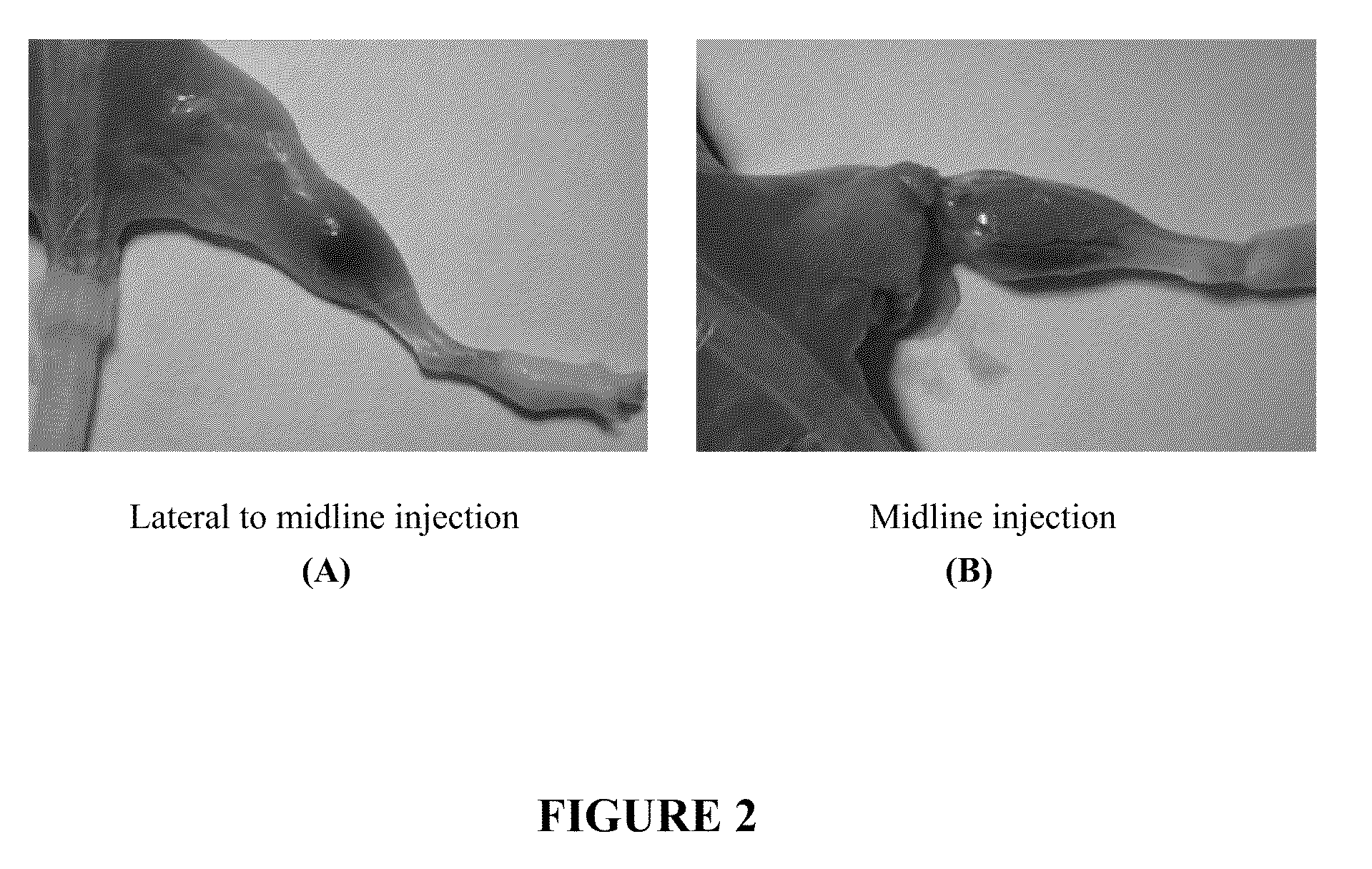
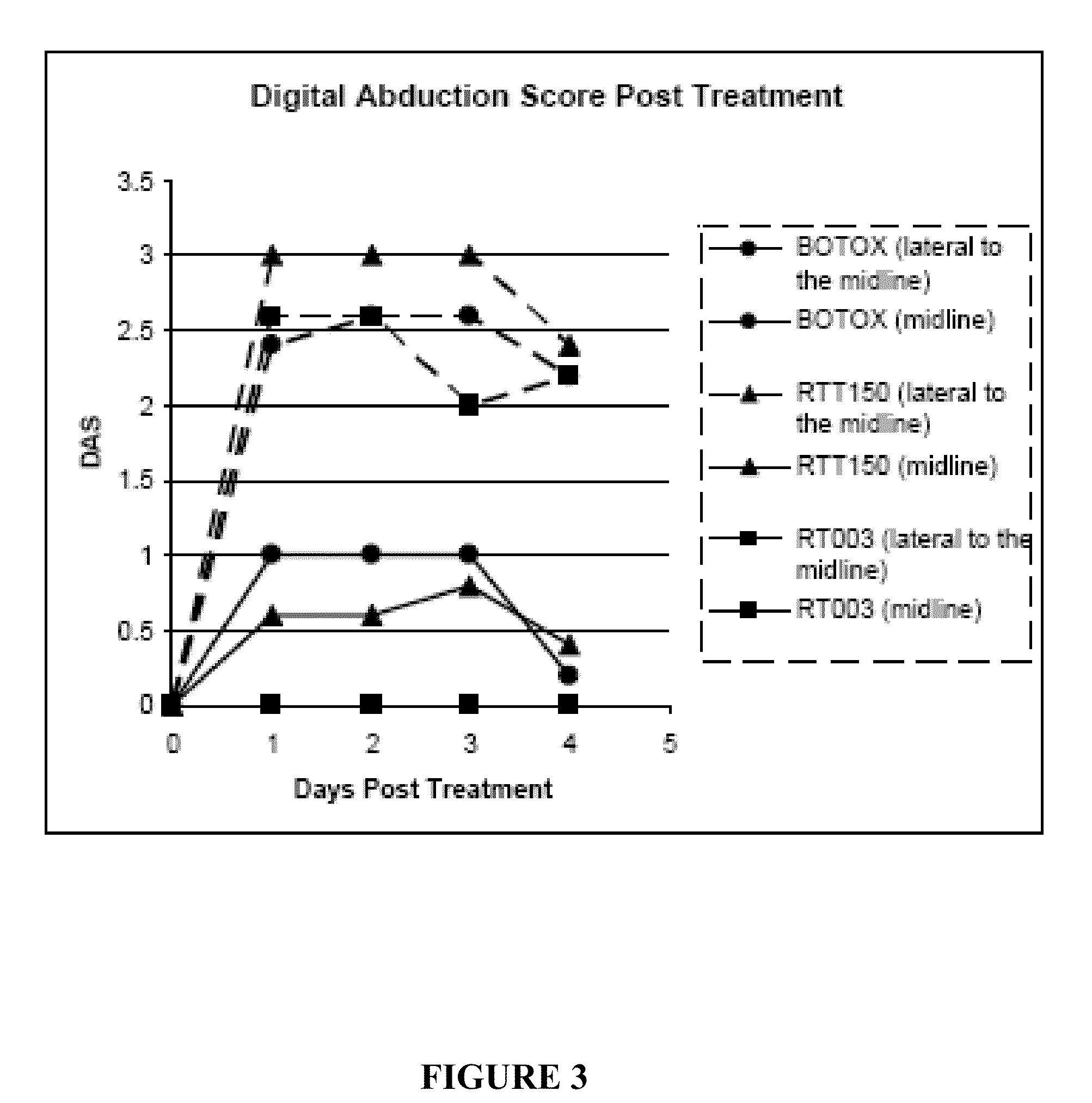
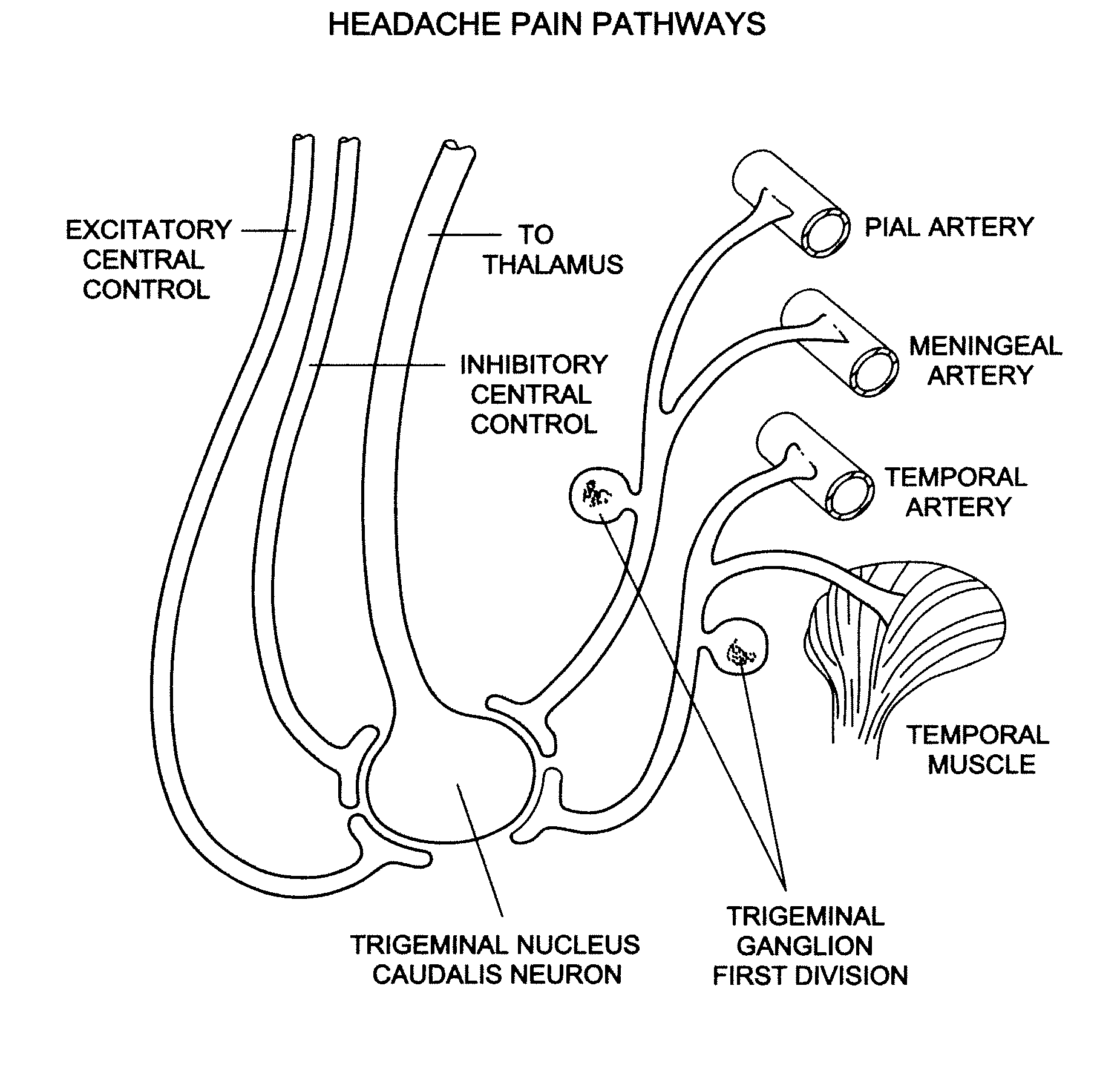
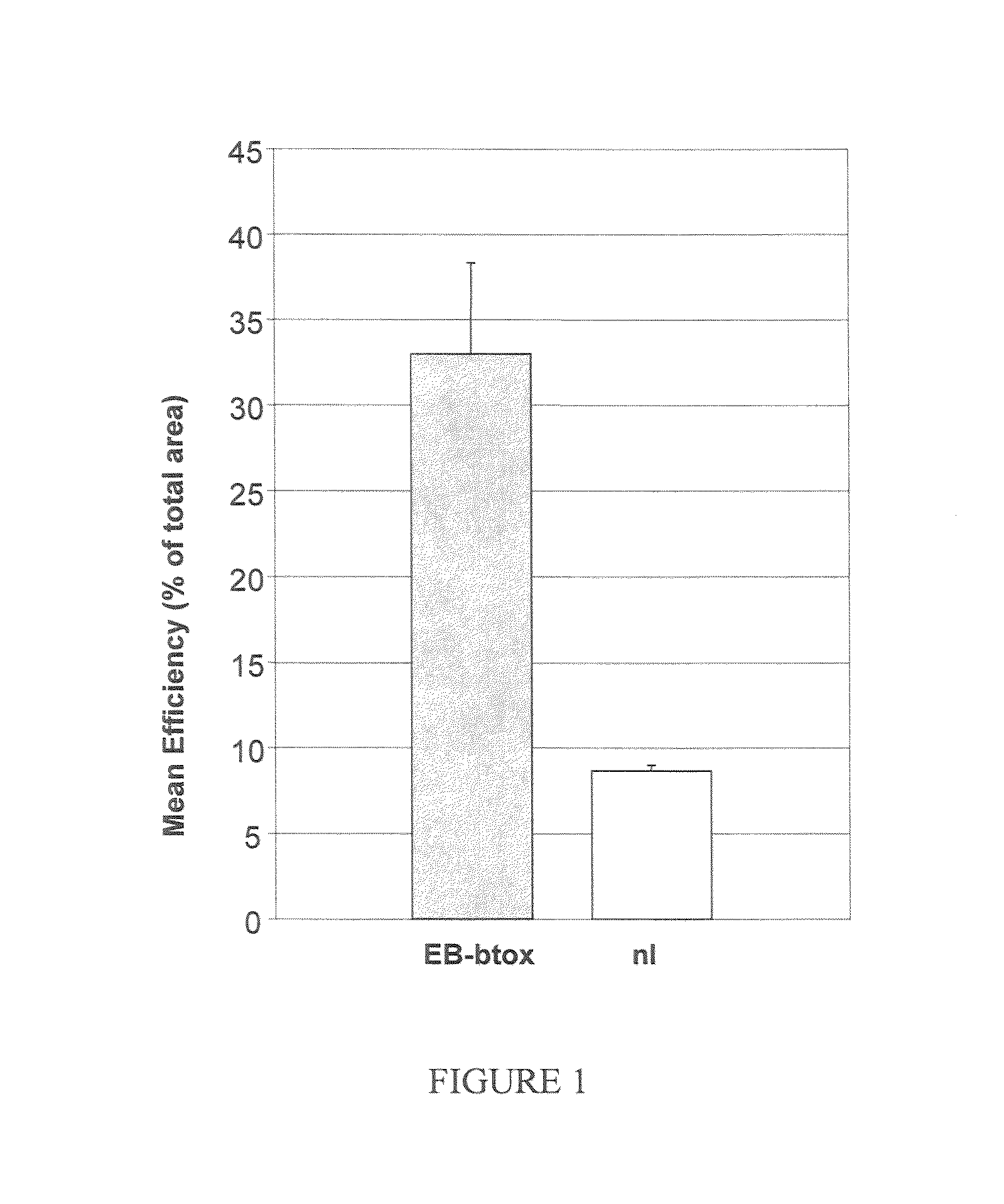
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Botulinum toxin a" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Botulinum Toxin Type A. Botulinum toxin type A is an injectable medicine used to treat a variety of conditions, from wrinkles and frown lines to severe underarm sweating to crossed eyes. The drug is actually a toxin produced by the Clostridium botulinum bacteria, and it works by decreasing the nerve signals that are sent to muscles.
Compositions and methods for topical application and transdermal delivery of botulinum toxins
ActiveUS20050196414A1Reduce hypersecretionReduce sweatingCosmetic preparationsSenses disorderEpitheliumMedicine
A composition for topical application of a botulinum toxin (including botulinum toxin derivatives) comprises a botulinum toxin and a carrier comprising a polymeric backbone comprising a long-chain polypeptide or nonpeptidyl polymer having attached positively charged branching or “efficiency” groups. The invention also relates to methods for reducing muscle paralysis and other conditions that may be treated with a botulinum toxin, particularly paralysis of subcutaneous, and most particularly, facial, muscles, by topically applying an effective amount of the botulinum toxin and carrier, in conjunction, to the subject's skin or epithelium. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Method for treating neuromuscular disorders and conditions with botulinum toxin types A and B
A method of treating a patient suffering from a disease, disorder or condition includes the administration to the patient of a therapeutically effective amount of botulinum toxin of a selected serotype until the patient experiences loss of clinical response to the administered botulinum toxin and thereafter administering to the patient a therapeutically effective amount of another botulinum toxin of a different serotype.
Owner:SOLSTICE NEUROSCI
Method for treating muscle spasm with botulinum toxin type B
A method and composition for treating a patient suffering from a disease, disorder or condition and associated pain include the administration to the patient of a therapeutically effective amount of a neurotoxin selected from a group consisting of Botulinum toxin types A, B, C, D, E, F and G.
Owner:SOLSTICE NEUROSCI
Botulinum toxin a peptides and methods of predicting and reducing immunoresistance to botulinum toxin therapy
ActiveUS20040265935A1Preventing and ameliorating intoxicationEffectively block effectBacterial antigen ingredientsPeptide/protein ingredientsOnabotulinum toxinToxin
The present invention provides BoNT / A peptides as well as methods of predicting or determining immunoresistance to botulinum toxin therapy in an individual using BoNT / A peptides.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Methods of administering botulinum toxin
InactiveUS7255865B2Easy transferCosmetic preparationsBacterial antigen ingredientsSmooth muscleMedicine
Methods for treating conditions in an animal or human subject. The conditions may be pain, skeletal muscle conditions, smooth muscle conditions, glandular conditions and cosmetic conditions. The methods comprise the step of administering a Clostridium neurotoxin component or Clostridium neurotoxin component encoding DNA to the subject using a needleless syringe.
Owner:ALLERGAN INC
Dermal Delivery
InactiveUS20160213757A1Not induce unwanted clinical effects inside and/orEnsure adequate treatmentCosmetic preparationsPeptide/protein ingredientsHypopigmentationTherapeutic effect
The present invention describes systems and methods for treating disorders and / or conditions associated with the dermal level of the skin. Such disorders include acne, hyperhidrosis, bromhidrosis, chromhidrosis, rosacea, hair loss, dermal infection, and / or actinic keratosis. Methods generally involve administering nanoemulsions (e.g., nanoparticle compositions) comprising at least one therapeutic agent, such as botulinum toxin. In some embodiments, nanoemulsions are prepared, e.g., by high pressure microfluidization, and comprise a particle size distribution exclusively between 10 nm and 300 nm.
Owner:ANTERIOS INC
Injectable Botulinum Toxin Formulations
InactiveUS20100168023A1Low antigenicityExtended durationCosmetic preparationsSenses disorderClinical efficacyBotulinum toxin type
This invention provides novel injectable compositions comprising botulinum toxin that may be administered to a subject for various therapeutic, aesthetic and / or cosmetic purposes. The injectable compositions contemplated by the invention exhibit one or more advantages over conventional botulinum toxin formulations, including reduced antigenicity, a reduced tendency to undergo unwanted localized diffusion following injection, increased duration of clinical efficacy or enhanced potency relative, faster onset of clinical efficacy, and / or improved stability.
Owner:REVANCE THERAPEUTICS INC
Botulinum toxin for treating postherpetic neuralgia
InactiveUS20090041805A1Easy transferCosmetic preparationsSenses disorderClostridial NeurotoxinIndividual animal
Methods for treating conditions in an animal or human subject. The conditions may be pain, skeletal muscle conditions, smooth muscle conditions, glandular conditions and cosmetic conditions. The methods comprise the step of administering a Clostridium neurotoxin component or Clostridium neurotoxin component encoding DNA to the subject using a needleless syringe.
Owner:ALLERGAN INC
Botulinum toxin in the treatment or prevention of acne
InactiveUS7226605B2Bacterial antigen ingredientsPeptide/protein ingredientsAnti-Androgen EffectMedicine
Botulinum toxin may be used to inhibit the cascade of events leading to acne. Results in preliminary studies have been dramatic. Without wishing to be bound by this theory, it is believed that botulinum toxin achieves this result through parasympathetic effects, inhibiting sweat gland activity, stimulating keratinocyte locomotion, anti-inflammatory effects, and possibly anti-androgenic effects. Botulinum toxin can play an important role in decreasing and even preventing the formation of acne.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
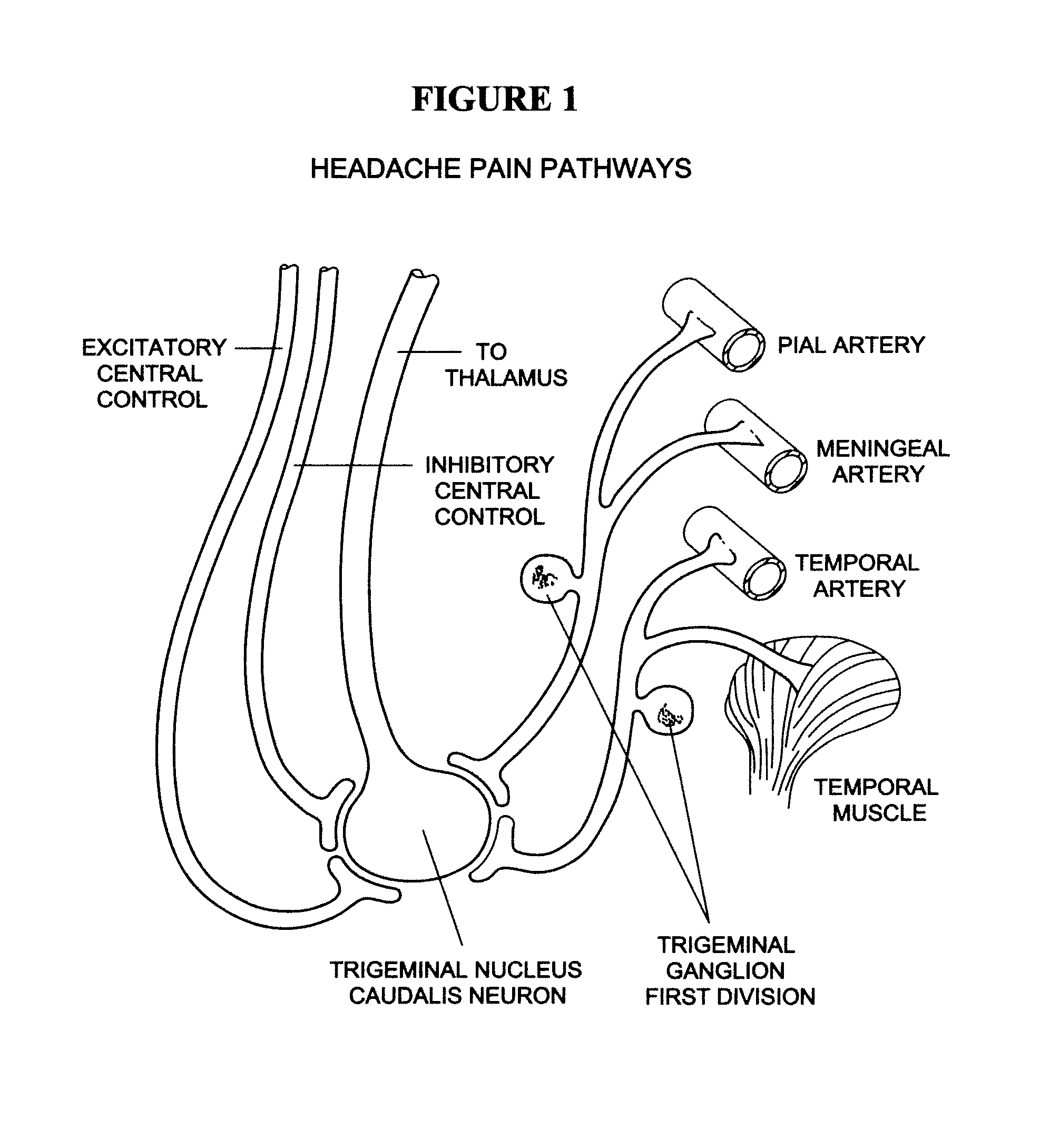
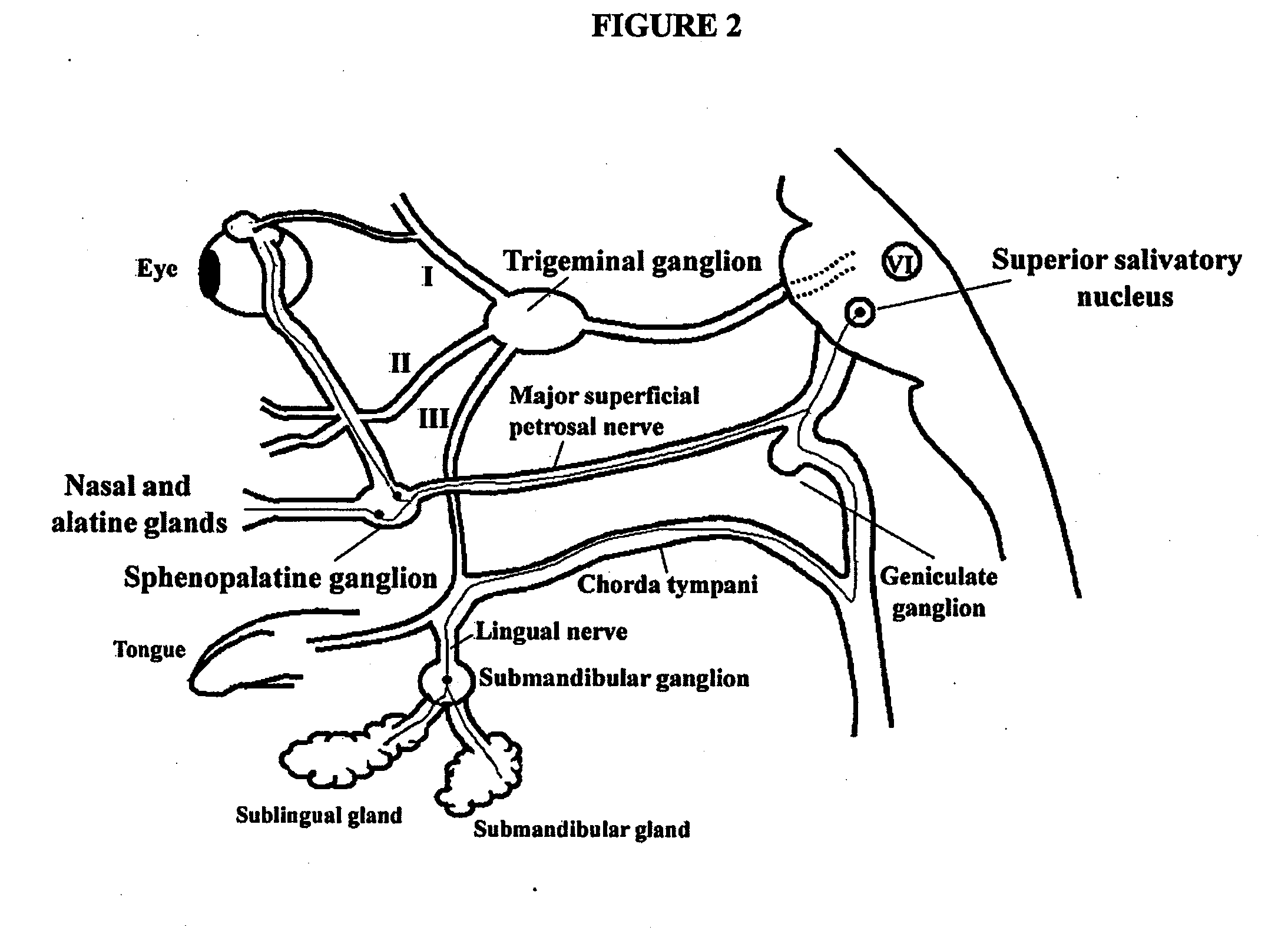
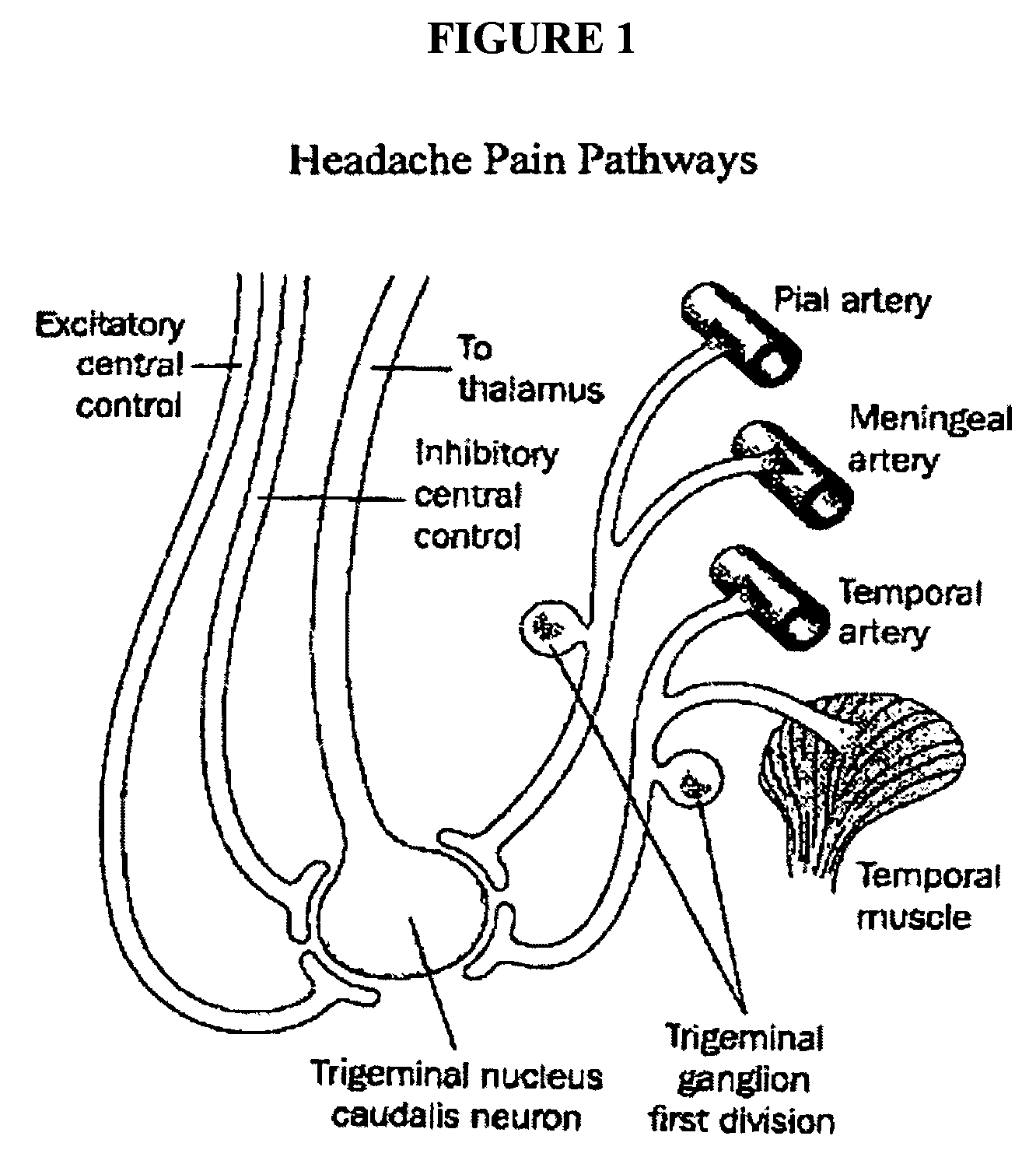
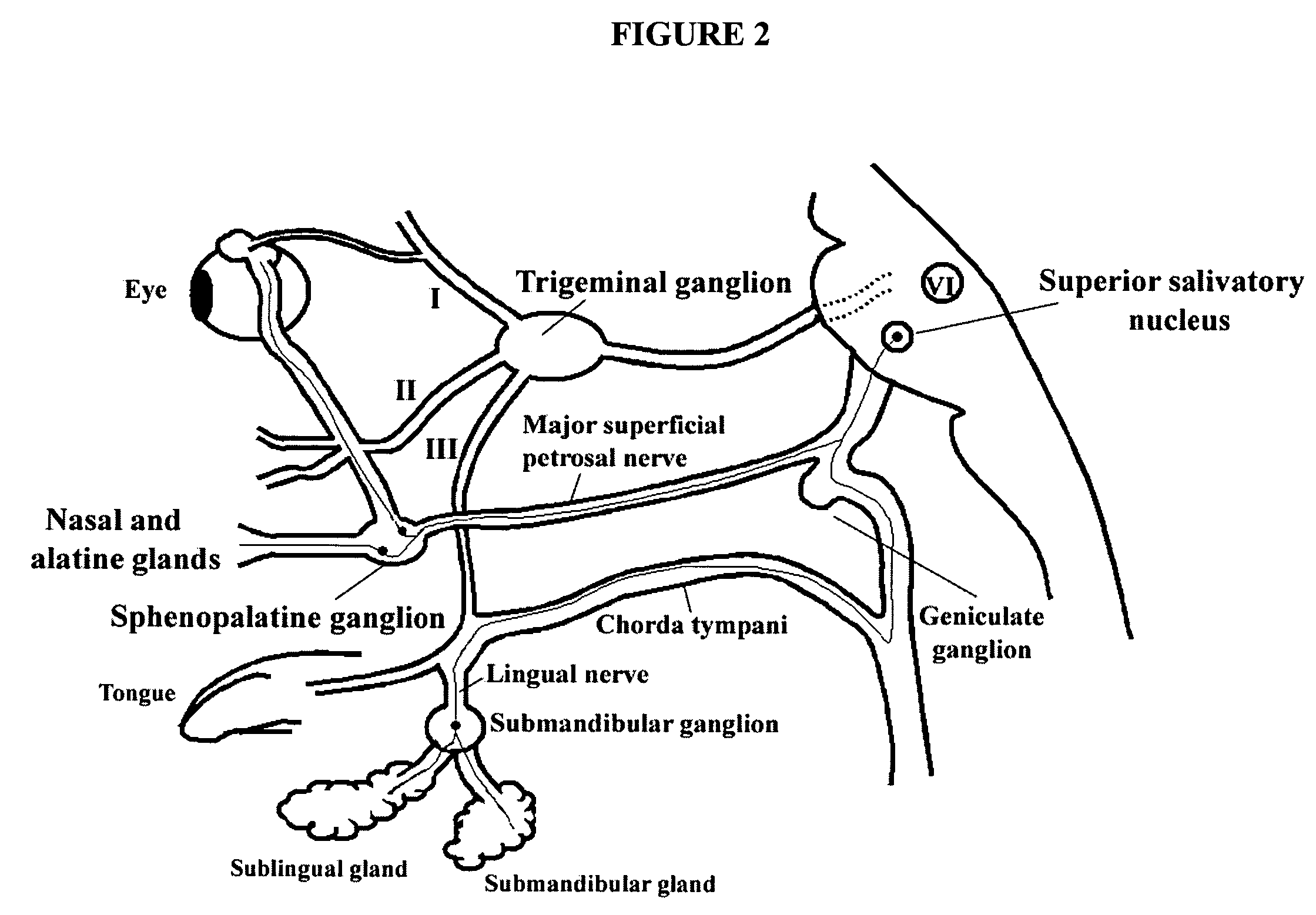
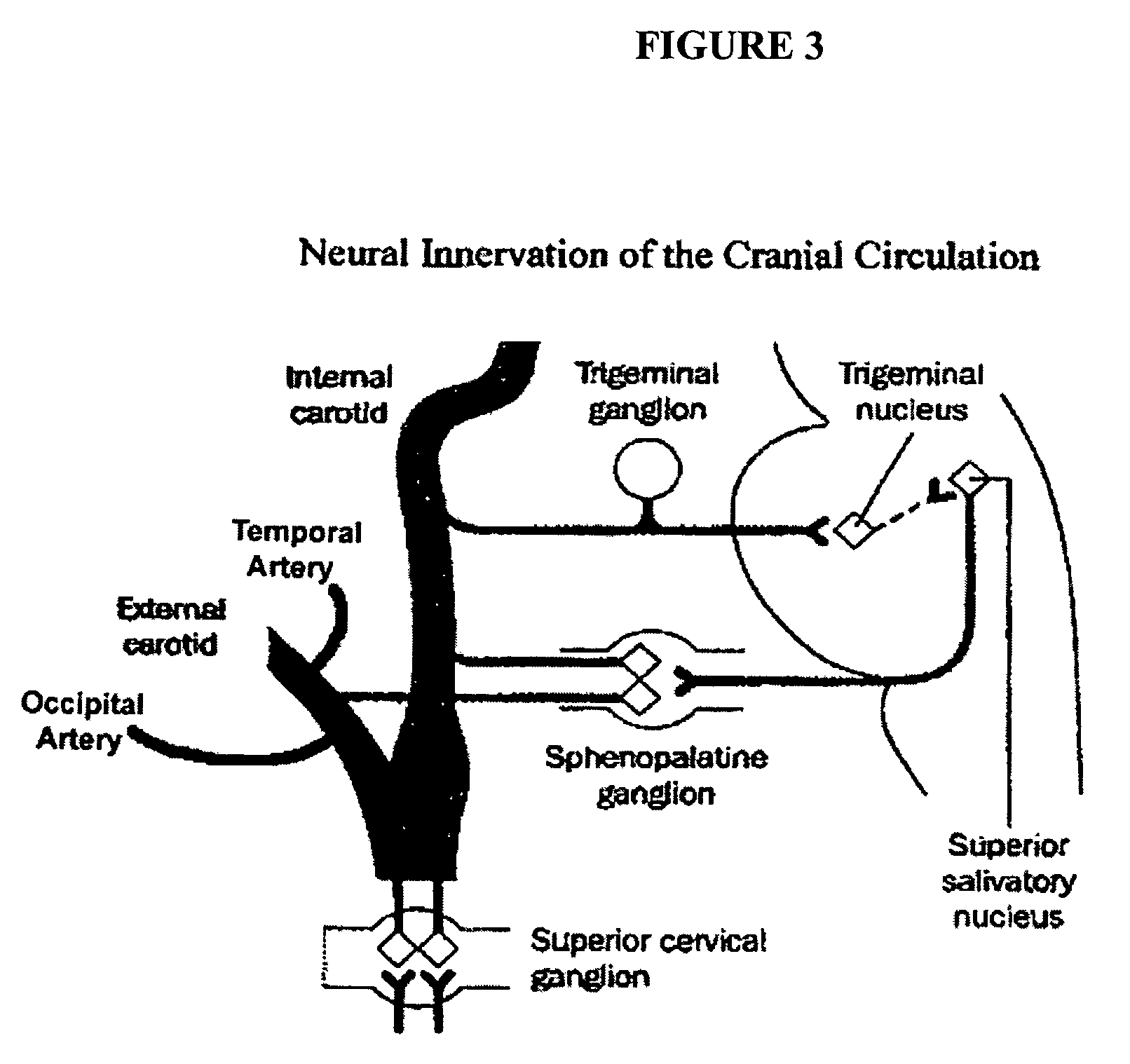
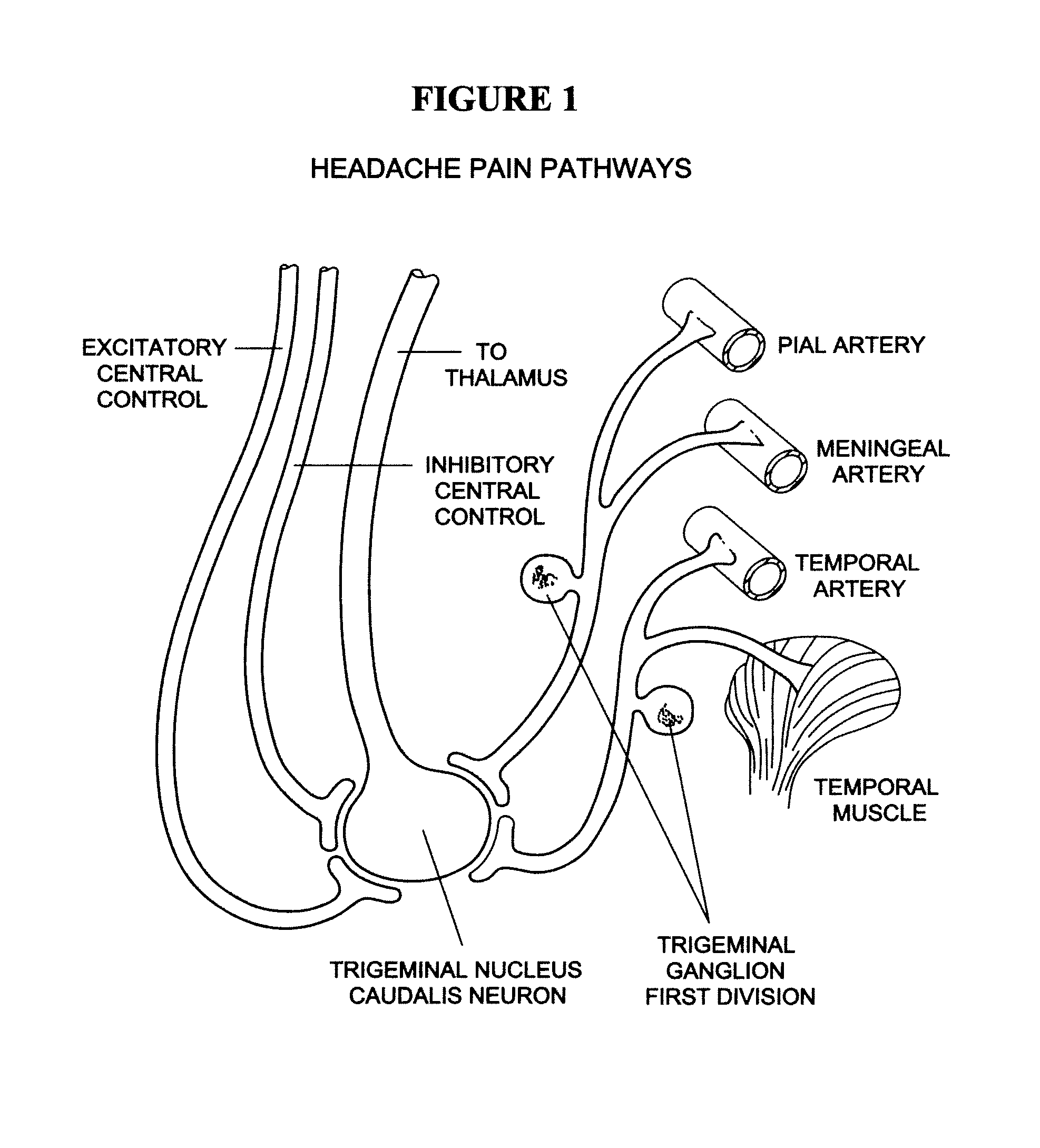
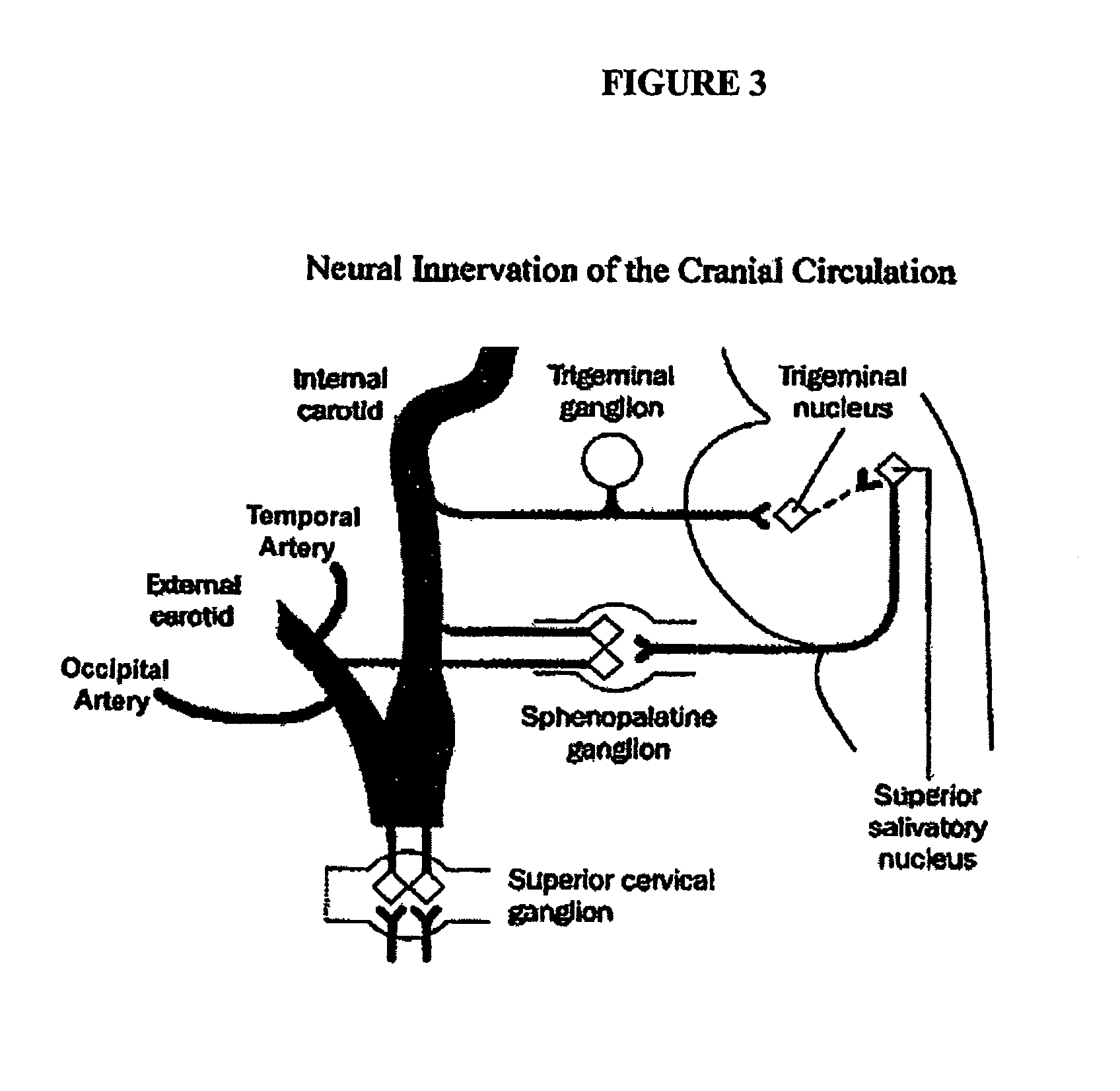
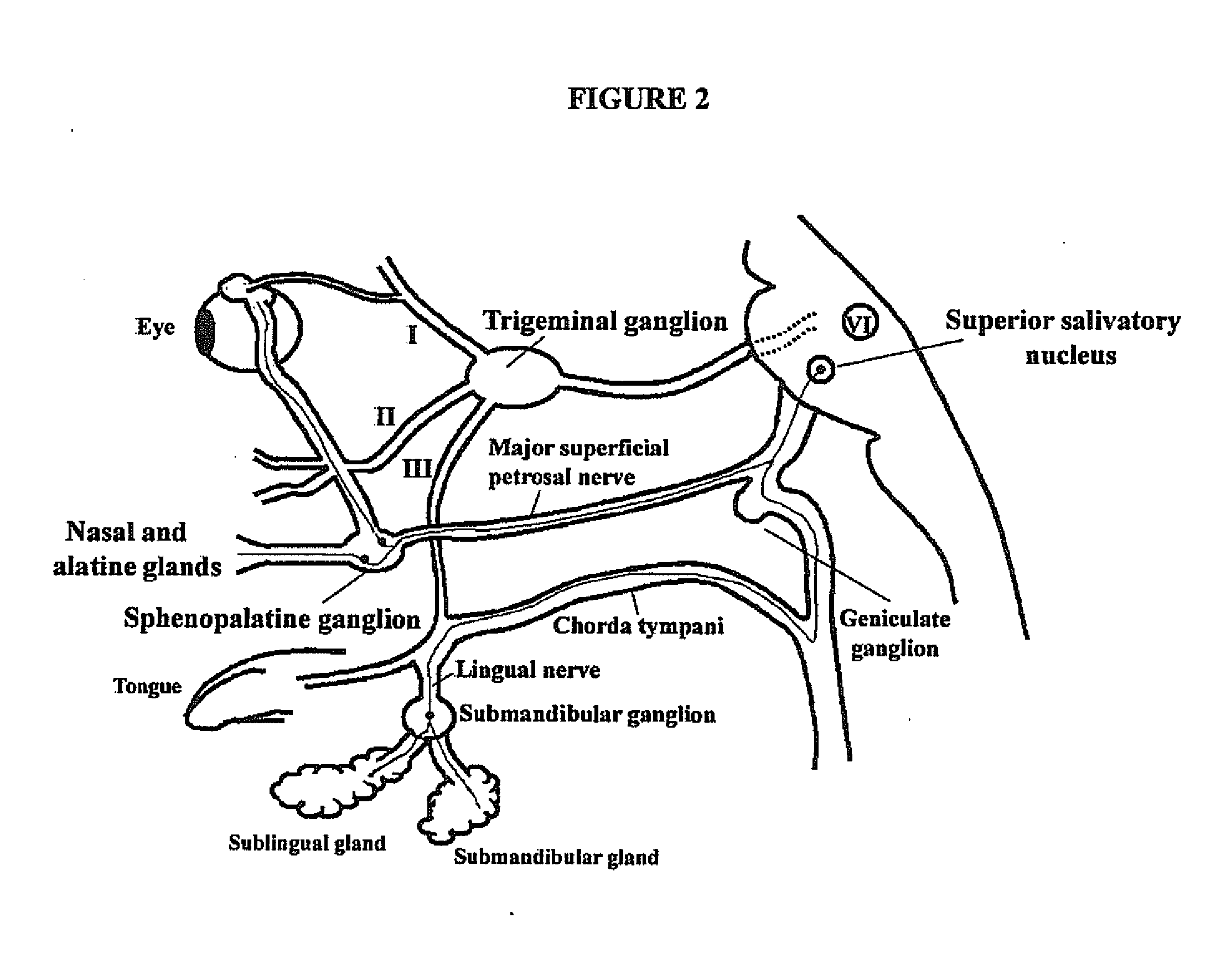
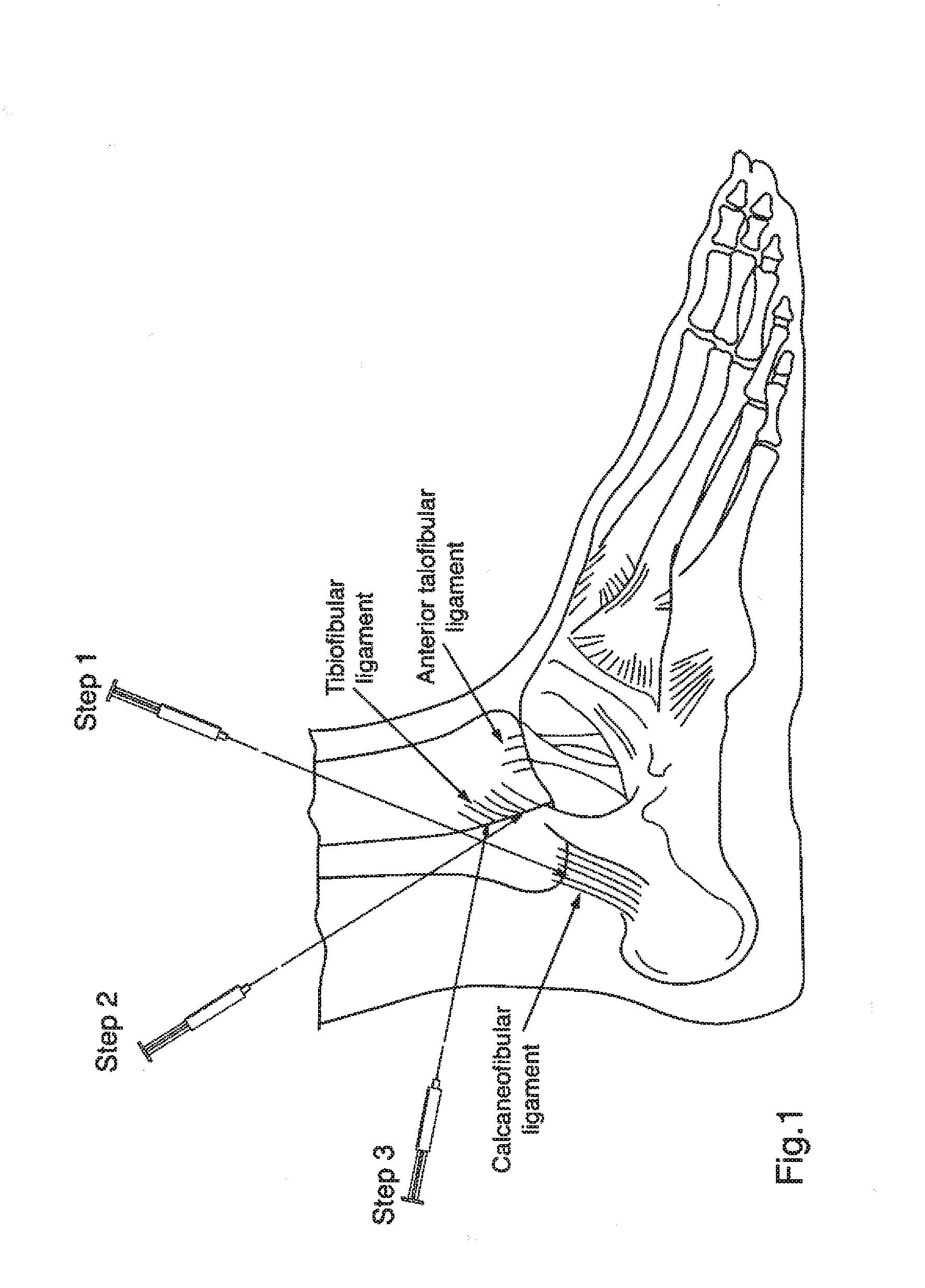
Targeted delivery of botulinum toxin to the sphenopalatine ganglion
ActiveUS20080279895A1Effective prophylactic treatmentReducing and preventing symptomNervous disorderBacteriaSynapseVascular disease
Owner:ALLERGAN INC
Targeted delivery of botulinum toxin for the treatment and prevention of trigeminal autonomic cephalgias, migraine and vascular conditions
ActiveUS7655244B2Effective treatmentReducing and preventing symptomBacterial antigen ingredientsNervous disorderSynapseVascular disease
Botulinum toxin, among other presynaptic neurotoxins is used for the treatment and prevention of migraine and other headaches associated with vascular disorders. Presynaptic neurotoxins are delivered focally, targeting the nerve endings of the trigeminal nerve, the occipital nerve and the intranasal terminals of the parasympathetic fibers originating in the Sphenopalatine ganglion. The administration preferably targets the extracranial nerve endings of the trigeminal nerve in the temporal area, the extracranial occipital nerve endings in the occipital area, and the intranasal terminals of the trigeminal nerve and parasympathetic fibers originating in the Sphenopalatine ganglion. The delivery is carried out by way of injection or topically.
Owner:ALLERGAN INC
Compositions and Methods for Topical Application and Transdermal Delivery of Botulinum Toxins
ActiveUS20080107690A1Reduce hypersecretionReduce sweatingOrganic active ingredientsCosmetic preparationsEpitheliumMedicine
Owner:REVANCE THERAPEUTICS INC
Use of botulinum toxins for treating various disorders and conditions and associated pain
A method and composition for treating a patient suffering from a disease, disorder or condition and associated pain include the administration to the patient of a therapeutically effective amount of a neurotoxin selected from a group consisting of Botulinum toxin types A, B, C, D, E, F and G.
Owner:ALLERGAN INC
Botulinum nanoemulsions
ActiveUS20120164182A1Without significant irritationCosmetic preparationsNervous disorderHigh pressureMimetic Muscles
The embodiment described herein are related nanoemulsions comprising botulinum toxins. In one embodiment, the nanoemulsions are prepared by high pressure microfluidization and comprise a particle size distribution exclusively between 10 and 300 nm. The nanoemulsions contemplated by the present invention are useful for the cosmetic and medical treatment of muscular contracture states. For example, botulinum toxin may relax facial muscles such that skin wrinkles become smoother and less noticeable. Further, the present invention contemplates a cosmetic formulation that may be self-administered, for example, in the privacy of one's home and without medical supervision.
Owner:UNIVERSITY OF MASSACHUSETTS LOWELL
Stable Formulations of Botulinum Toxin in Hydrogels
InactiveUS20120238504A1Reduced responsivenessImprovement in patientHormone peptidesCosmetic preparationsMedicineTreatment use
The invention includes liquid formulations of botulinum toxin, including hydrogel formulations that are stable to storage in liquid form at standard refrigerator temperatures for at least 1-2 years and to storage at higher temperatures for at least 6 months. The invention also includes methods of treatment using such formulations for various therapeutic and cosmetic purposes.
Owner:SOLSTICE NEUROSCI
Targeted delivery of botulinum toxin to the sphenopalatine ganglion
Botulinum toxin, among other presynaptic neurotoxins is used for the treatment and prevention of migraine and other headaches associated with vascular disorders. Presynaptic neurotoxins are delivered focally, targeting the sphenopalatine ganglion. Exemplary delivery is carried out by way of injection.
Owner:ALLERGAN INC
Botulinum toxin and the treatment of primary disorders of mood and affect
ActiveUS8926991B2Reduce transmissionRelieve symptomsBacterial antigen ingredientsPeptide/protein ingredientsBotulinum toxin typeClinical psychology
The invention provides methods for treating primary disorders of mood and affect, including depressive disorders, anxiety and sleep disorders and CNS disorders comprising the administration of a neurotoxin.
Owner:REVANCE THERAPEUTICS INC
Lyophilized preparation of botulinum toxin
ActiveUS8920795B2Good storage stabilityMaintain stabilityPowder deliverySenses disorderMedicineToxin
There are provided a lyophilized preparation of botulinum toxin without a protein stabilizer derived from animals. The lyophilized preparation of botulinum toxin according to the present invention can maintain an activity of botulinum toxin, and also exhibit excellent long-term storage stability even under conditions of high temperature, which may occur when botulinum toxin is stored, delivered, and processed.
Owner:MEDY TOX INC
Lyophilized Preparation of Botulinum Toxin
ActiveUS20140086900A1Good storage stabilityMaintain stabilityPowder deliverySenses disorderMedicineToxin
There are provided a lyophilized preparation of botulinum toxin without a protein stabilizer derived from animals. The lyophilized preparation of botulinum toxin according to the present invention can maintain an activity of botulinum toxin, and also exhibit excellent long-term storage stability even under conditions of high temperature, which may occur when botulinum toxin is stored, delivered, and processed.
Owner:MEDY TOX INC
High frequency application of botulinum toxin therapy
ActiveUS8557255B2High frequencyRaise the possibilityCosmetic preparationsBacterial antigen ingredientsDiseaseHigh doses
The present invention relates to methods for treating diseases and disorders by administering a composition containing the neurotoxic component of a Clostridium botulinum toxin complex, wherein the composition is devoid of any other protein of the Clostridium botulinum toxin complex and wherein the composition is administered at short intervals and / or in high doses.
Owner:MERZ PHARMA GMBH & CO KGAA
Compositions and methods for topical application and transdermal delivery of botulinum toxins
ActiveUS9211248B2Reduce hypersecretionReduce sweatingPolypeptide with localisation/targeting motifBiocideEpitheliumMedicine
A composition for topical application of a botulinum toxin (including botulinum toxin derivatives) comprises a botulinum toxin and a carrier comprising a polymeric backbone comprising a long-chain polypeptide or nonpeptidyl polymer having attached positively charged branching or “efficiency” groups. The invention also relates to methods for reducing muscle paralysis and other conditions that may be treated with a botulinum toxin, particularly paralysis of subcutaneous, and most particularly, facial, muscles, by topically applying an effective amount of the botulinum toxin and carrier, in conjunction, to the subject's skin or epithelium. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
Process for providing a temperature-stable muscle relaxant on the basis of the neurotoxic component of botulinum toxin in a solid form
The present invention relates to a method for providing a muscle relaxant at temperatures above 20° C., wherein said muscle relaxant is a solid dry composition comprising the neurotoxic component of botulinum toxin free of complexing proteins.
Owner:MERZ PHARMA GMBH & CO KGAA
Treatment of chronic chalazion and hordeolum with botulinum toxin
ActiveUS7335367B2Reduce secretionAntibacterial agentsBacterial antigen ingredientsCulture mediumsInspissation
Chalazia and hordeola are the most common lesions occurring in the human eyelid, and often recurrences are managed by surgical intervention to remove fatty inclusions within the lid with associated inflammatory reaction. The present invention provides non-surgical methods of treating chalazia, hordeola and cutaneous infections comprising the administration of compositions comprising botulinum toxin. The present invention provides methods that effectively block meibum secretion from the meibomian glands, reduce sebaceous bacterial culture media on skin, and sebaceous secretion from the glands of Zeis. Decreased production of meibum and associated fatty substances resulting from the methods of the present invention, decrease gland blockage and tissue inspissations, resulting in reduced recurrence of chalazia, hordeola and related inflammatory reactions and lesions.
Owner:REVANCE THERAPEUTICS INC
Use of an Acmella oleracea extract for the botulinum toxin-like effect thereof in an anti-wrinkle cosmetic composition
Spilanthol, in the form of an Acmella oleracea extract, inhibits contractions in subcutaneous muscles, notably those of the face, and can be used as an anti-wrinkle product. A cosmetic treatment procedure for wrinkles consists of locally or subcutaneously applying an effective quantity of a composition based on spilanthol pure or in the form of an Acmella oleracea extract.
Owner:GATTEFOSSE SA
Targeted delivery of botulinum toxin to the sphenopalatine ganglion
Botulinum toxin, among other presynaptic neurotoxins is used for the treatment and prevention of migraine and other headaches associated with vascular disorders. Presynaptic neurotoxins are delivered focally, targeting the sphenopalatine ganglion. Exemplary delivery is carried out by way of injection.
Owner:ALLERGAN INC
Method of treating skin needing botulinum toxin type a treatment
InactiveUS20070154493A1Improve efficiencyExtended durationCosmetic preparationsBacterial antigen ingredientsBotulinum toxin typeCosmetic procedures
A treatment regimen for treating skin subject to a botulinum toxin type A cosmetic procedure involves the application of supplemental composition(s) such as preparatory composition(s), protective composition(s), and combinations thereof, and a corrective composition.
Owner:HATTENDORF JUDY +1
Treatment of autism using botulinum toxins
A method of treating ASD (autism) in a patient in need thereof comprises administering a botulinum toxin to the patient. The botulinum toxin may be administered by subcutaneous / intradermal injection. The subcutaneous / intradermal injection may be administered to and / or around the vicinity of a trigeminal nerve, a cervical nerve, a thoracic nerve, a lumbar nerve, and a sacral nerve of the patient. In infants or toddlers—from about 1 to 5 year olds, it is used to prevent or minimize damage to the developing brain; in older children and adult Autism Spectrum Disorder (ASD) patients, it will be used to reduce or eliminate their symptoms.
Owner:PENLAND FOUND
Methods of using Botulinum toxin for the treatment of hypervolemic lip deformity (lip ectropion)
The present invention uses pharmaceutical preparations of Botulinum toxin to induce neurogenic atrophy to alter muscle volume and subsequently, facial contour. Reduction in lip volume is accomplished using the disclosed methods thereby producing a favorable effect on a hypervolemic lip deformity. In other embodiments of the invention, the methods disclosed herein may be used to shrink muscle bulk and contour, especially in the face. In one embodiment, treating excessive muscle bulk below the eyelids can cause a smoothing of the lower lid and cheek contour producing a favorable improvement in appearance. Such an application is in distinct contrast to rhytide (wrinkle reduction) as the injected region does not contain wrinkles or any other form of dynamic line, merely excessive tissue bulk.
Owner:REVANCE THERAPEUTICS INC
Compositions and Methods for Topical Application and Transdermal Delivery of Botulinum Toxins
ActiveUS20130202636A1Reduce hypersecretionReduce sweatingPolypeptide with localisation/targeting motifBacterial antigen ingredientsEpitheliumMedicine
A composition for topical application of a botulinum toxin (including botulinum toxin derivatives) comprises a botulinum toxin and a carrier comprising a polymeric backbone comprising a long-chain polypeptide or nonpeptidyl polymer having attached positively charged branching or “efficiency” groups. The invention also relates to methods for reducing muscle paralysis and other conditions that may be treated with a botulinum toxin, particularly paralysis of subcutaneous, and most particularly, facial, muscles, by topically applying an effective amount of the botulinum toxin and carrier, in conjunction, to the subject's skin or epithelium. Kits for administration are also described.
Owner:REVANCE THERAPEUTICS INC
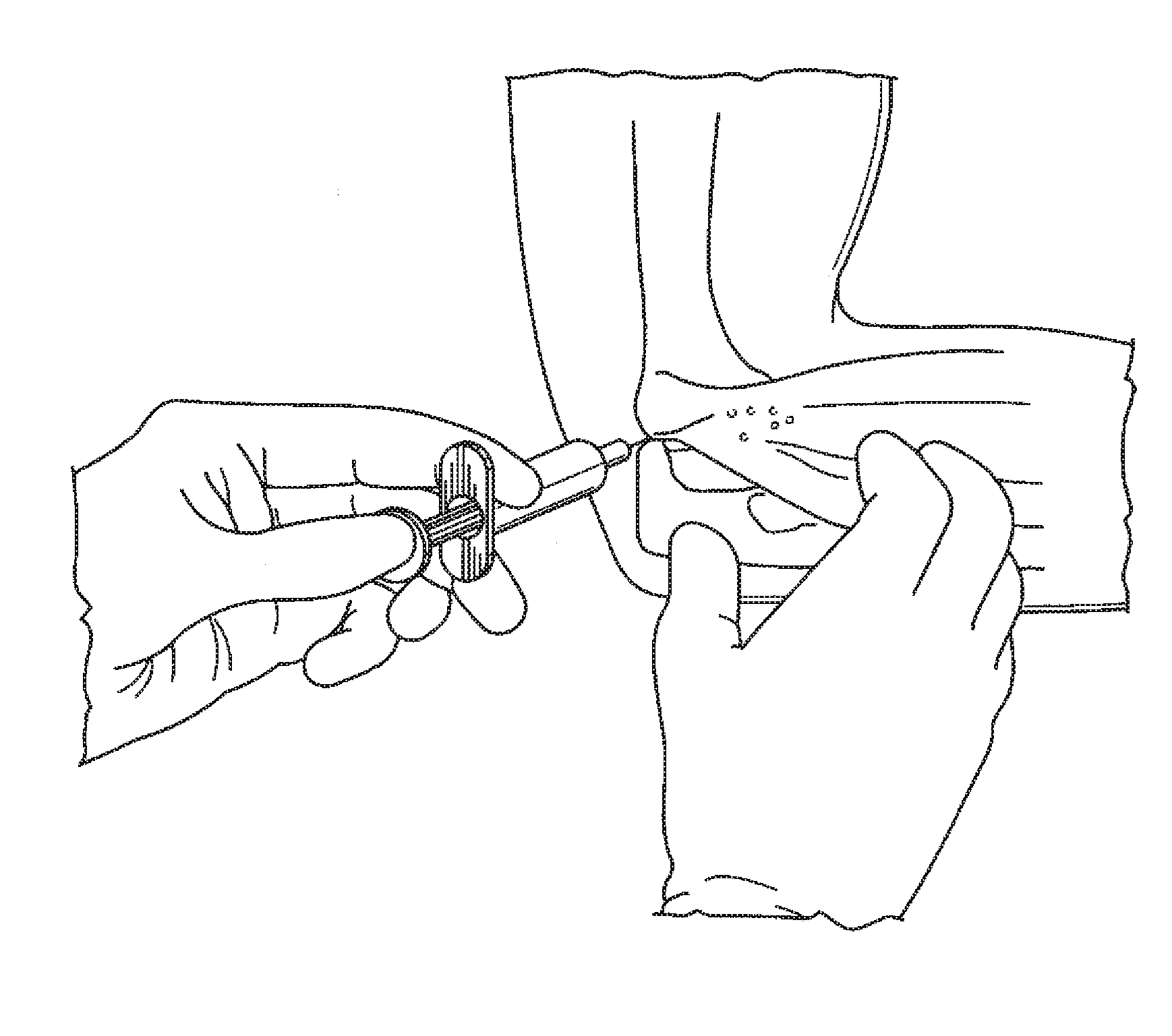
Treatment of soft tissue injury using hyaluronic acid and botulinum toxin
ActiveUS20100172940A1Potentiates healing effect of heatGood treatment effectOrganic active ingredientsBacterial antigen ingredientsMammalTreated animal
There is provided a method for supporting soft tissue in a mammal. The method may in aspects treat acutely or chronically injured soft tissue in an animal or human, the method comprising administering a therapeutically effective amount of HA and botulinum toxin in combination around the injured soft tissue. The method is useful for the treatment of sprain and strain in an animal such as a human.
Owner:2405871 ONTARIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com