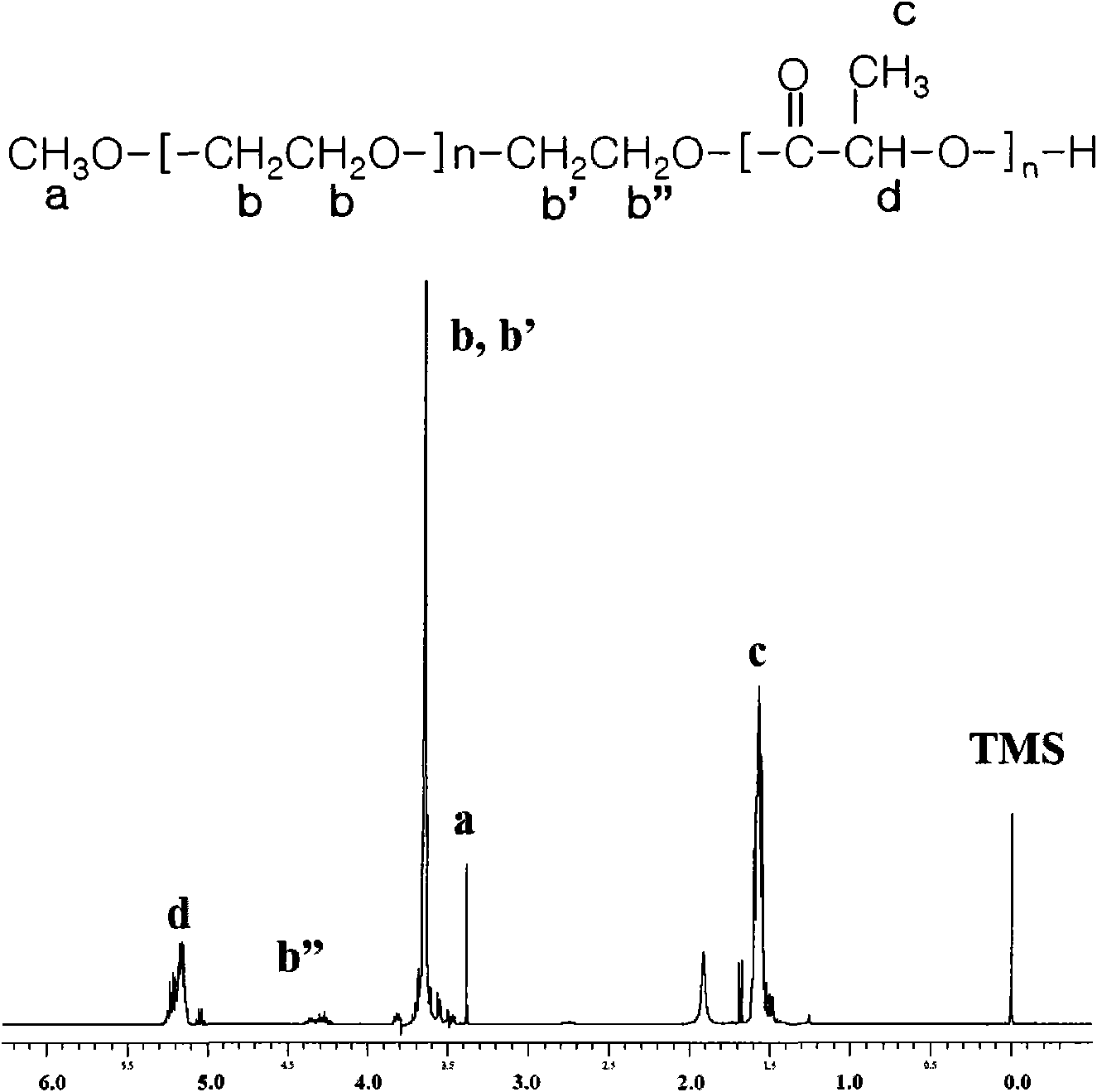Amphiphilic block copolymer micelle composition containing taxane and manufacturing process of the same
An amphiphilic block and composition technology, which is applied in the directions of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of reduced pharmacological effect, degradation of active pharmaceutical ingredients, etc., so as to reduce the preparation time and prevent rapid The best effect of release, pharmacological effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0052] Preparation Example 1: Monomethoxy polyethylene glycol-polylactide (mPEG-PLA) block copolymer (A-B type) synthesis
[0053] First, 5.0 g of monomethoxypolyethylene glycol (number average molecular weight: 2,000 Dalton) was introduced into a 100 mL two-necked round bottom flask, and heated at 130° C. for 3-4 hours under reduced pressure (1 mmHg) to remove water from it. Next, the flask was purged with nitrogen, and 0.1 wt% (10.13 mg, 25 mmol) of stannous octoate (Sn(Oct) 2 ) based on the weight of D-lactide and L-lactide was added thereto using a syringe. as a reaction catalyst. The reaction mixture was stirred for 30 minutes, then allowed to depressurize (1 mmHg) at 130° C. for 1 hour to remove the solvent (toluene) which dissolved the catalyst. Then, 10.13 g of purified lactide was added thereto, and the resulting mixture was heated at 130° C. for 18 hours. After heating, the resulting polymer was dissolved in dichloromethane, and this was added to diethyl ether...
preparation example 2
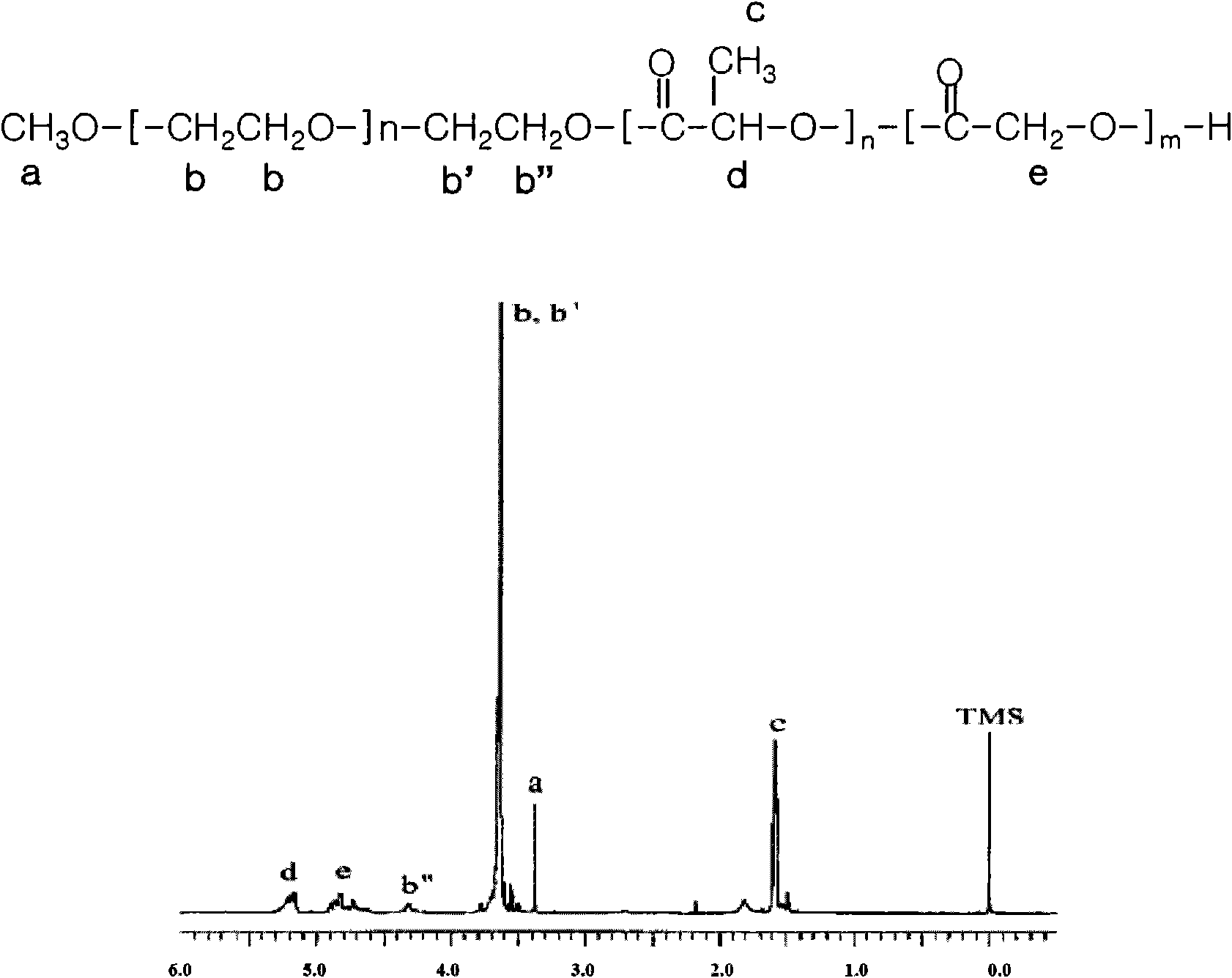
[0055] Preparation Example 2: Monomethoxy polyethylene glycol-polylactic acid-glycolic acid copolymer (mPEG-PLGA) block Synthesis of Copolymer (Type A-B)
[0056] In the same manner as in Preparation Example 1, by making monomethoxypolyethylene glycol (number average molecular weight: 5,000 Daltons), lactide and glycolide in the presence of stannous octoate as a catalyst, The reaction was carried out at 120° C. for 12 hours to obtain a block copolymer.
[0057] The block copolymer monomethoxy polyethylene glycol-polylactic acid-glycolic acid copolymer (mPEG-PLGA) has a number average molecular weight of 5,000-4,000 Daltons and is an A-B type copolymer. pass 1 Analysis of the copolymer by H-NMR revealed that the copolymer is an A-B type diblock copolymer (see figure 2 ).
Embodiment 1
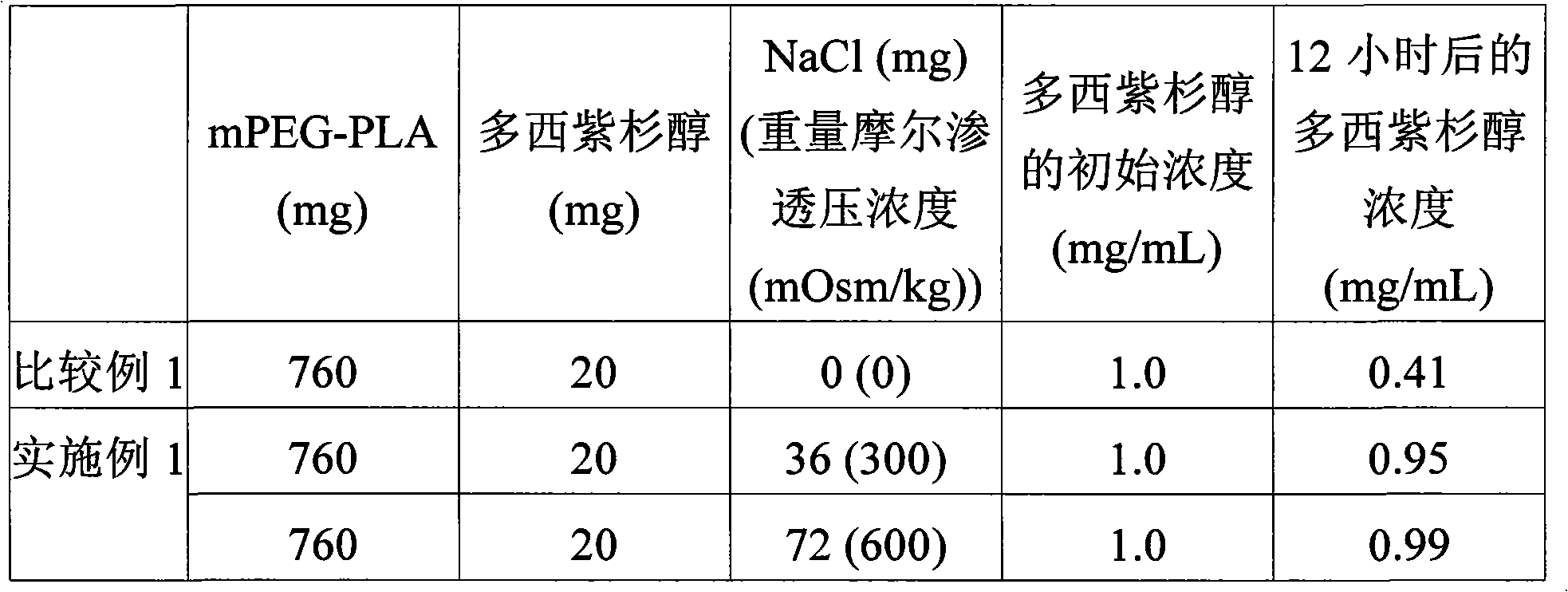
[0058] Example 1: mPEG-PLA block copolymer micelles containing sodium chloride and docetaxel compound preparation
[0059] First, 760 mg of the amphiphilic block copolymer mPEG-PLA (number average molecular weight: 2,000-1,765 Daltons) obtained in Preparation Example 1 was completely dissolved in 0.2 mL of ethanol at 60°C to provide clear ethanol solution. The ethanol solution was cooled to 25°C, and 20 mg of docetaxel was added thereto, and the resulting solution was stirred until docetaxel was completely dissolved.
[0060] Next, aqueous solutions each containing 0.9 wt % and 1.8 wt % of sodium chloride and having an osmolality of 300 mOsm / kg and 600 mOsm / kg were prepared in separate containers. The osmolarity was measured by using a commercially available osmometer (Gonotech GmbH, OSMOMAT030). 4 mL of each aqueous solution was added to the ethanol solution containing the copolymer, and the resulting mixture was stirred at 40° C. for 10 minutes to form an aqueous solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com