Method for testing a cell sample
a cell sample and cell technology, applied in the field of cell sample testing, can solve the problems of misdiagnosis and patient's death, method most often fails, and error is rarely recognised
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
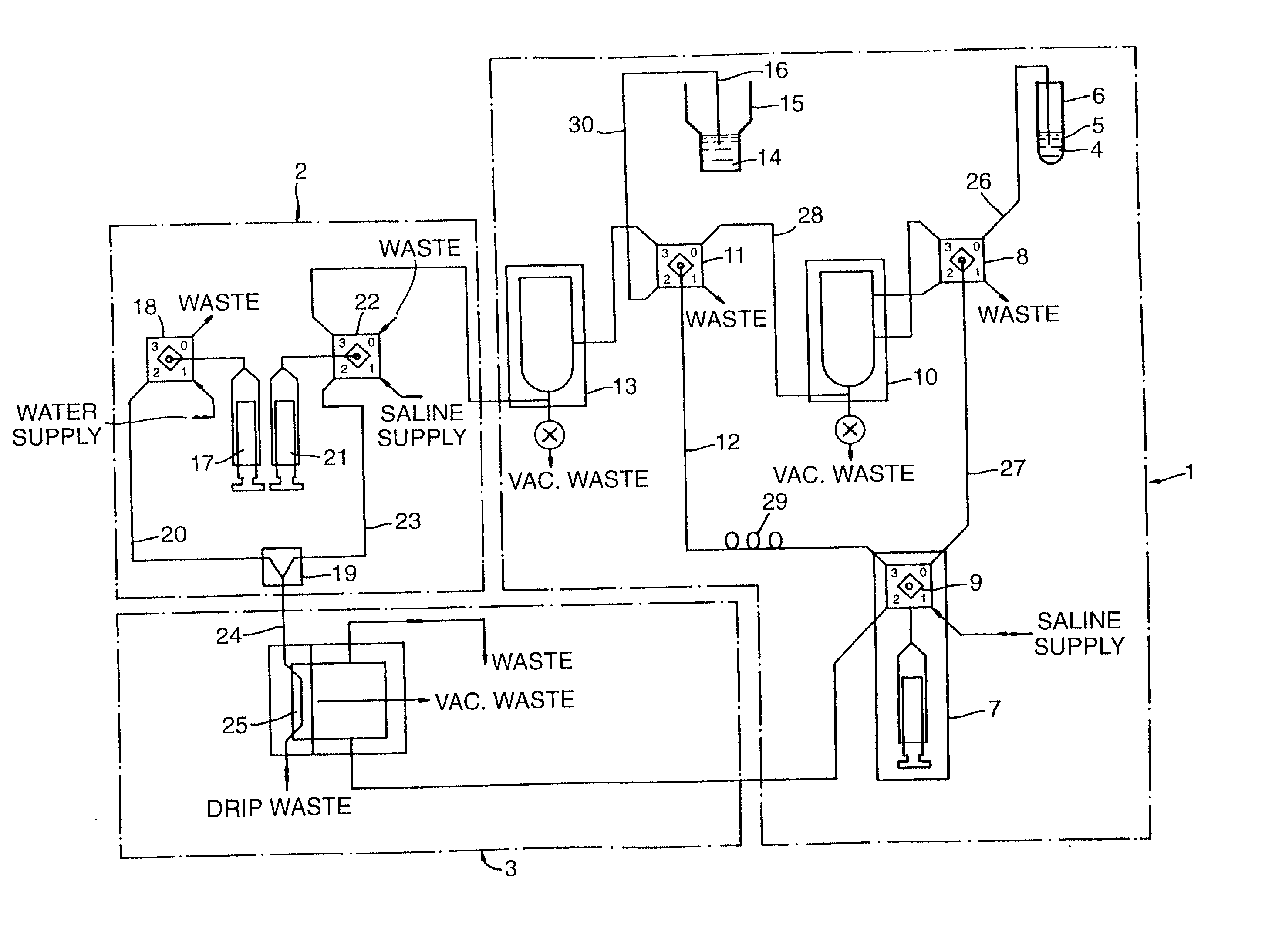
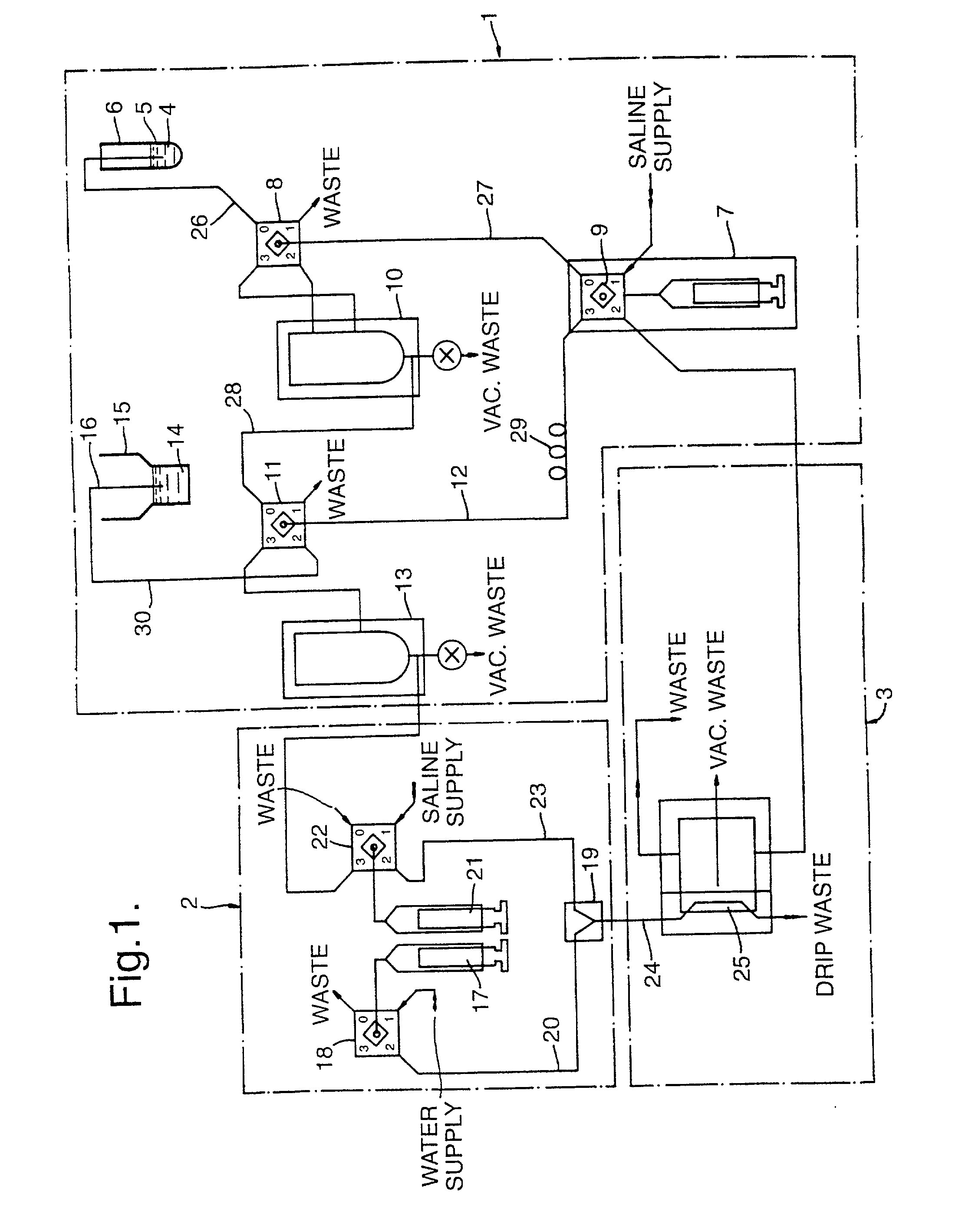
[0092] FIG. 1 shows schematically an instrument used to sample and test blood cells;
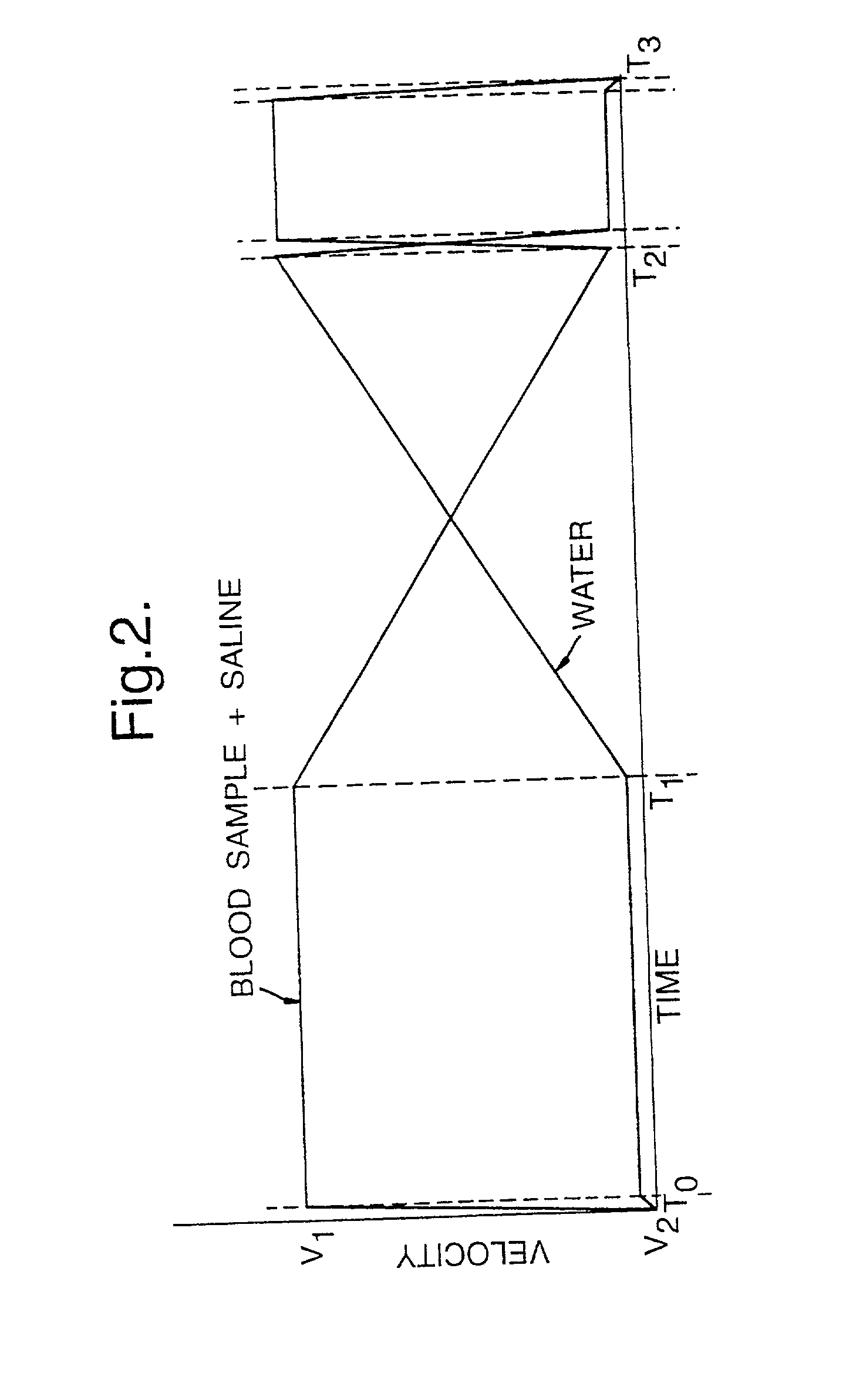
[0093] FIG. 2 shows velocity profiles for the discharge of fluids from fluid delivery syringes of a gradient generator section of the instrument of FIG. 1;
[0094] FIG. 3 shows a block diagram illustrating the data processing steps used in the instrument of FIG. 1;
[0095] FIG. 4 shows an example of a three-dimensional plot of osmolality against measured voltage for cells of a blood sample analyzed in accordance with the present invention;
[0096] FIG. 5 shows another example of a three-dimensional plot of osmolality against measured voltage which illustrates the frequency distribution of blood cells at intervals;
[0097] FIG. 6 shows a series of three-dimensional plots for a sample tested at hourly intervals;
[0098] FIGS. 7 and 8 show results for spherical latex particles as part of an instrument calibration routine;
[0099] FIG. 9 shows superimposed plots of osmolality (x-axis) against measured voltage and tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com