Method for controlling medicament release rate of orally disintegrating tablet
A technology for oral disintegrating tablets and disintegrating agents, which is applied in the field of oral disintegrating tablets, can solve the problems of unqualified product content uniformity, exceeding the disintegration time, and increasing the amount of binder, so as to achieve easy product quality, reduce The effect of slow release rate and reduced contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048]
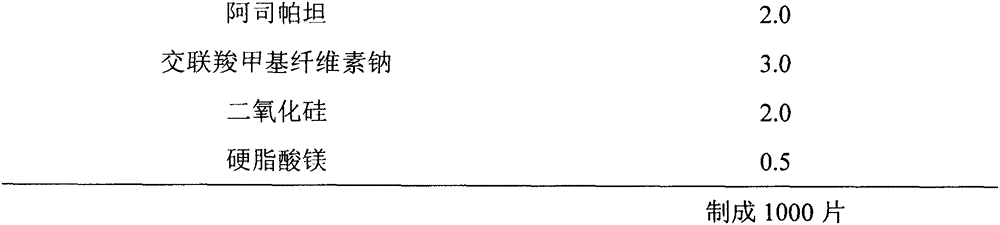
[0049] Preparation method: mix aripiprazole, microcrystalline cellulose, pregelatinized starch, mannitol, ethyl cellulose, and aspartame evenly, add appropriate amount of absolute ethanol to granulate, and dry in a fluidized bed to obtain dry granules The dry granules are uniformly mixed with croscarmellose sodium, silicon dioxide and magnesium stearate after granulation, and pressed into tablets.
[0050] The samples of comparative example 1 and embodiment 1 are all tested stripping curves with the same method (paddle method 50rpm, pH1.0 hydrochloric acid solution 900mL), and the results are as follows:
[0051] sample
Embodiment 2
[0053]
[0054] Preparation method: Mix aripiprazole, microcrystalline cellulose, pregelatinized starch, mannitol, ethyl cellulose, red iron oxide, and aspartame evenly, add appropriate amount of absolute ethanol to granulate, pass through a fluidized bed Dried to obtain dry granules, which are uniformly mixed with croscarmellose sodium, silicon dioxide and magnesium stearate after granulation, and pressed into tablets.
Embodiment 3
[0056]
[0057] Preparation method: dissolve ethyl cellulose in an appropriate amount of isopropanol, mix aripiprazole, anhydrous calcium hydrogen phosphate, pregelatinized starch, glucose, and sucralose evenly, add ethyl cellulose in isopropanol The solution is granulated, dried in a fluidized bed to obtain dry granules, and after granulation, the dry granules are evenly mixed with croscarmellose sodium, micropowder silica gel and magnesium stearate, and pressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com