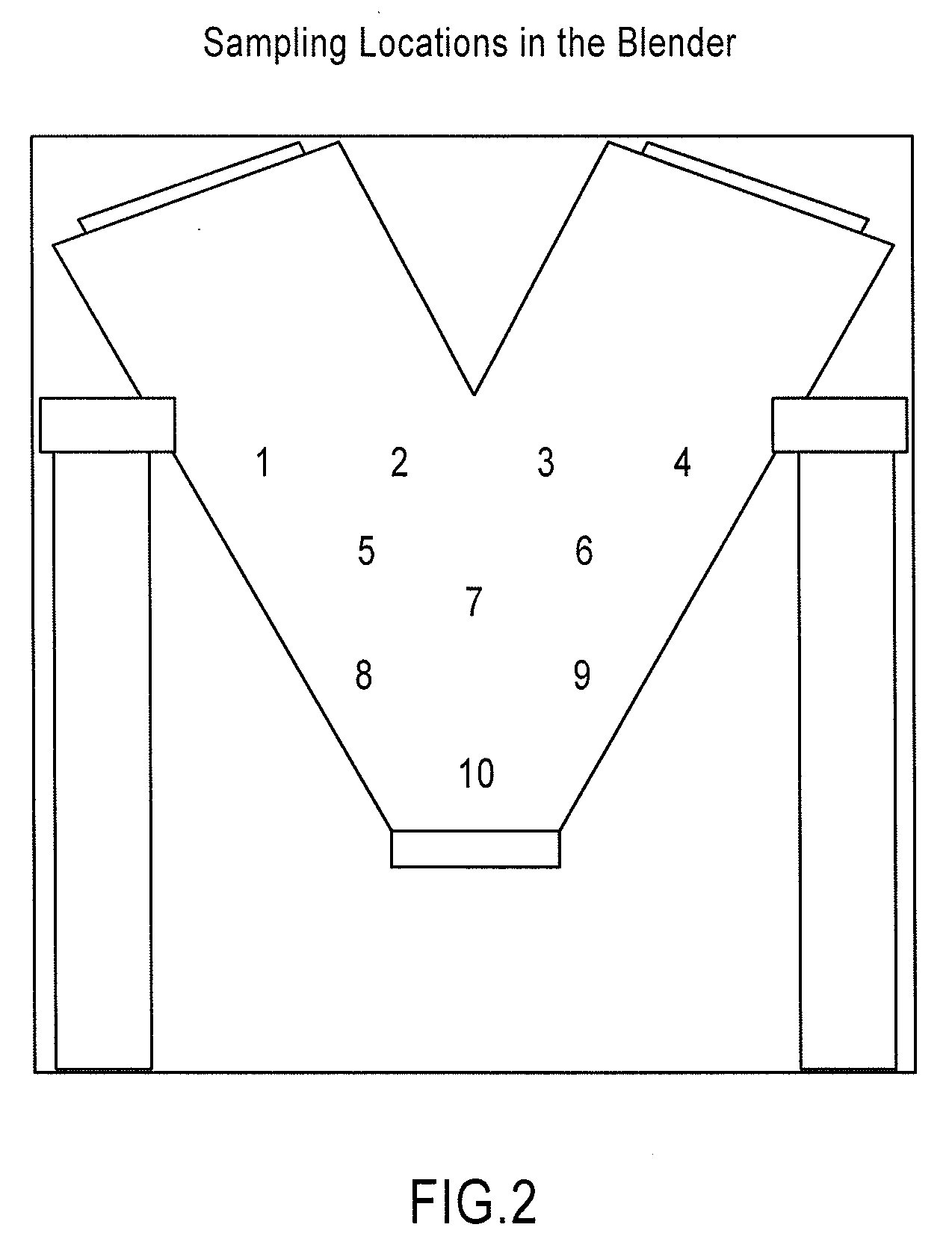
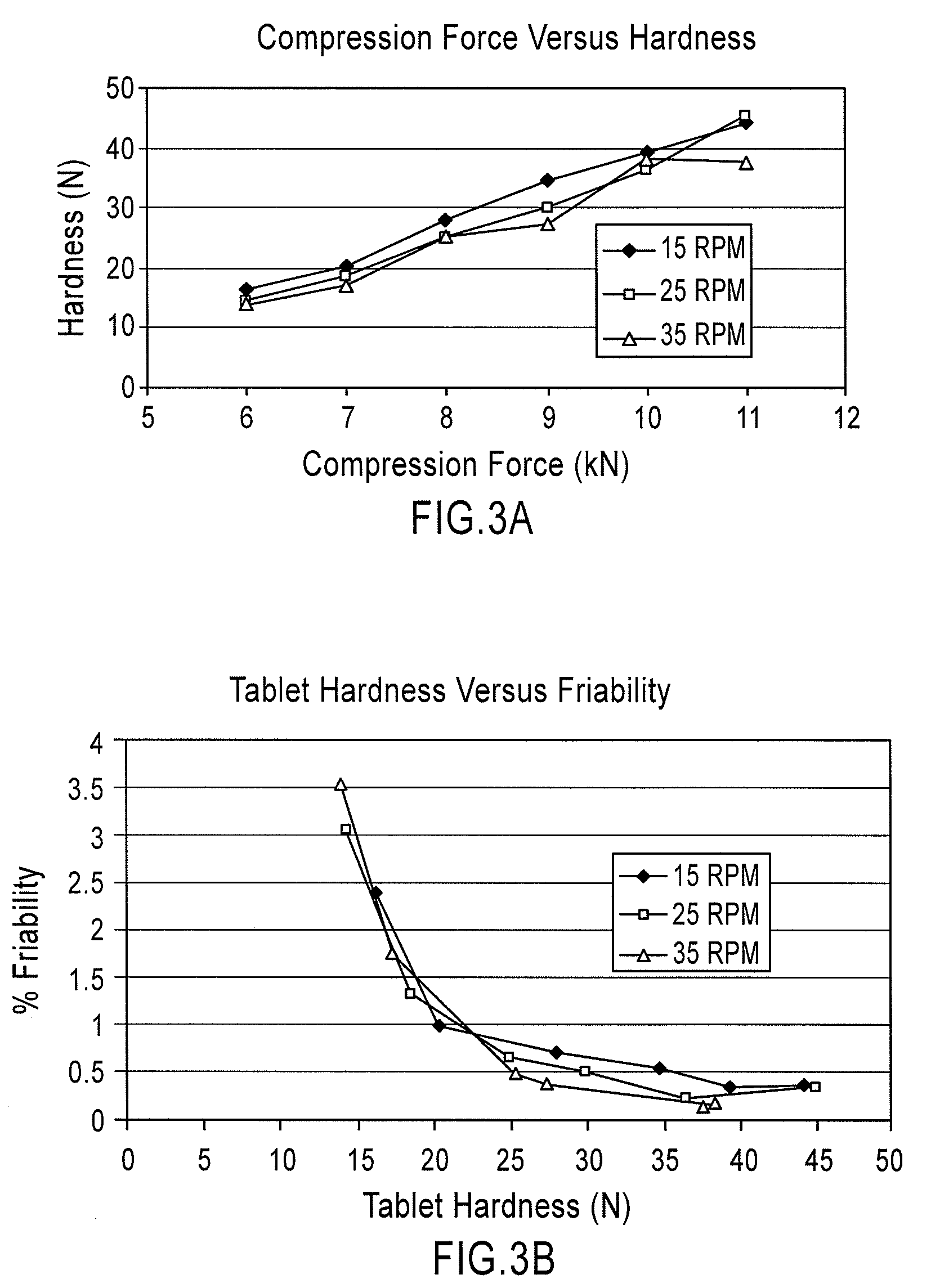
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Have Difficulty Swallowing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The medical term for difficulty swallowing is dysphagia, which affects more than 10 million adults in the U.S. according to the National Institute of Health. Dysphagia refers to all the reasons someone might have trouble swallowing.
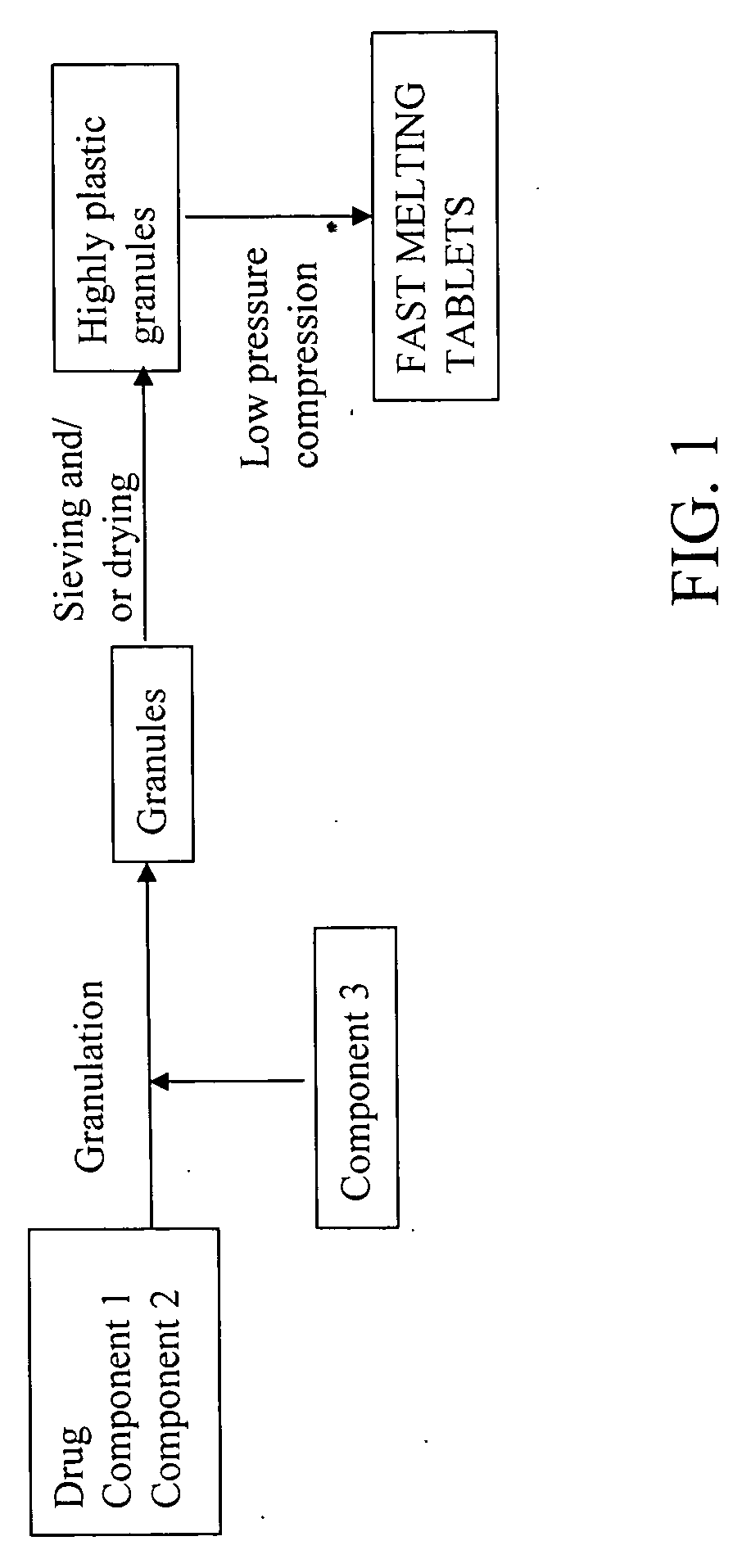
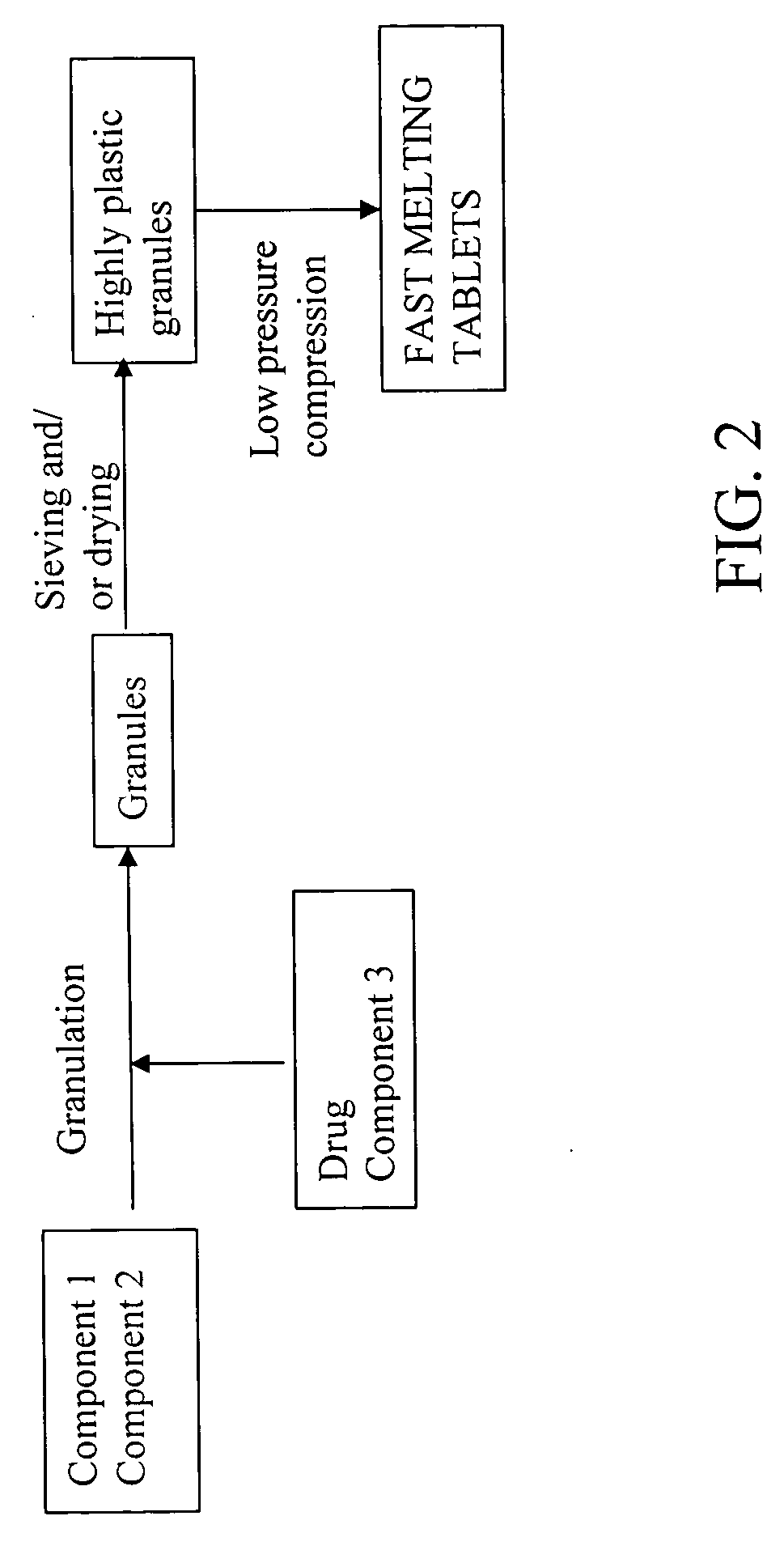
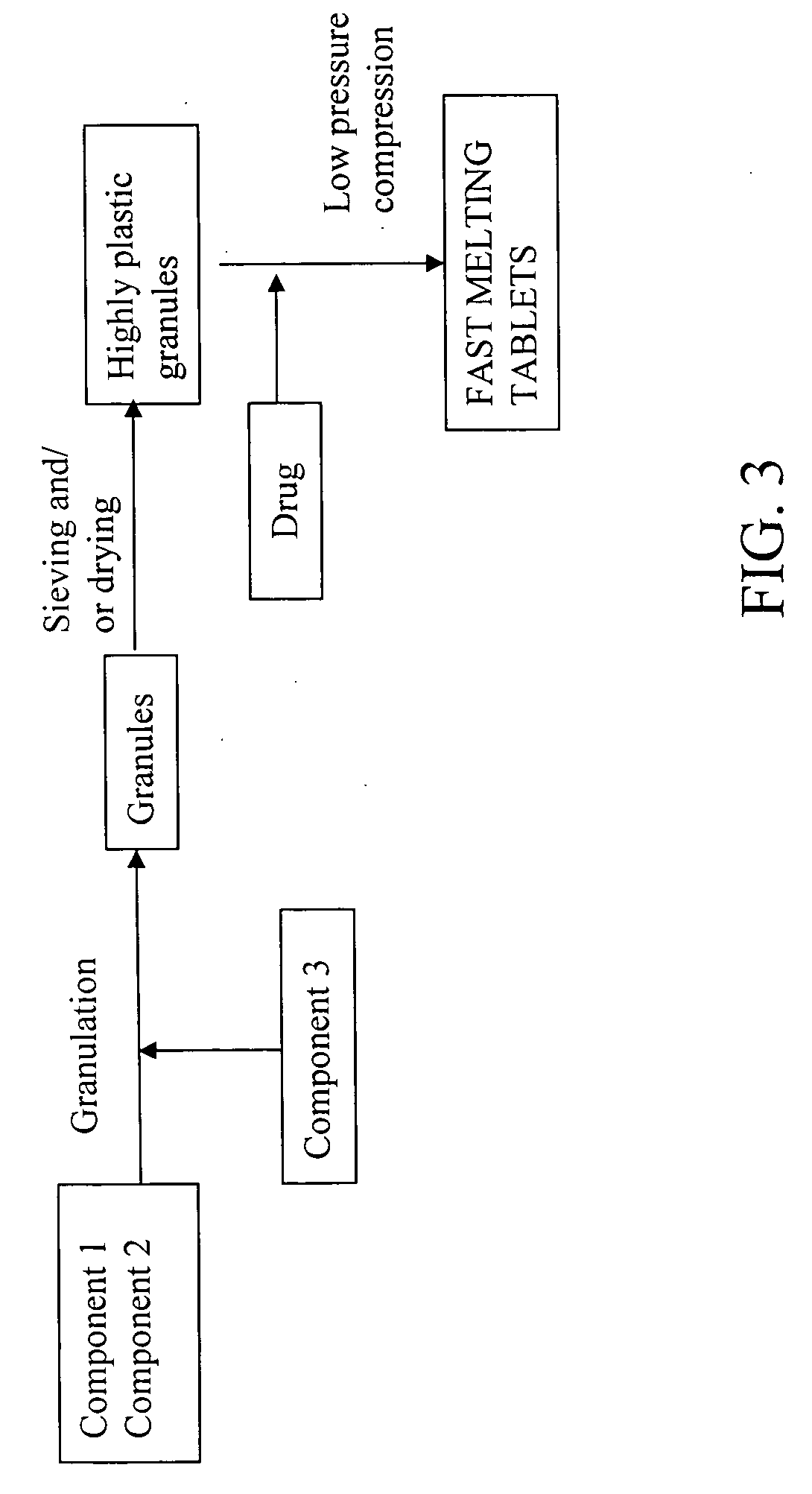
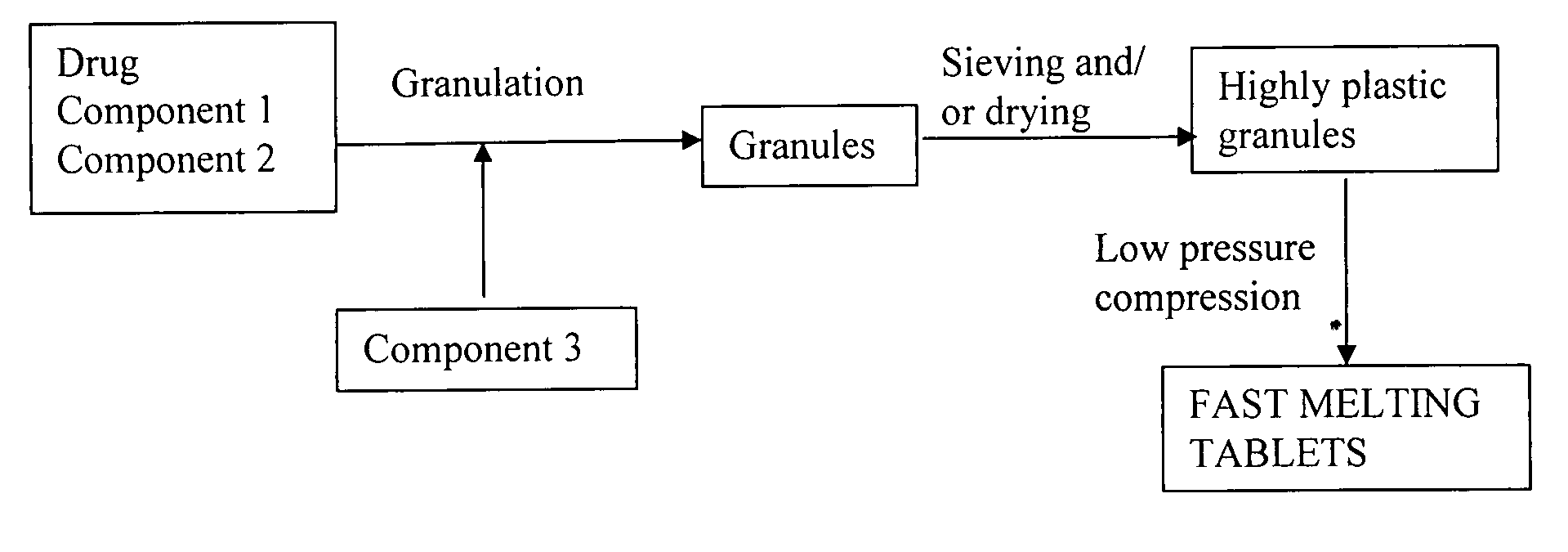
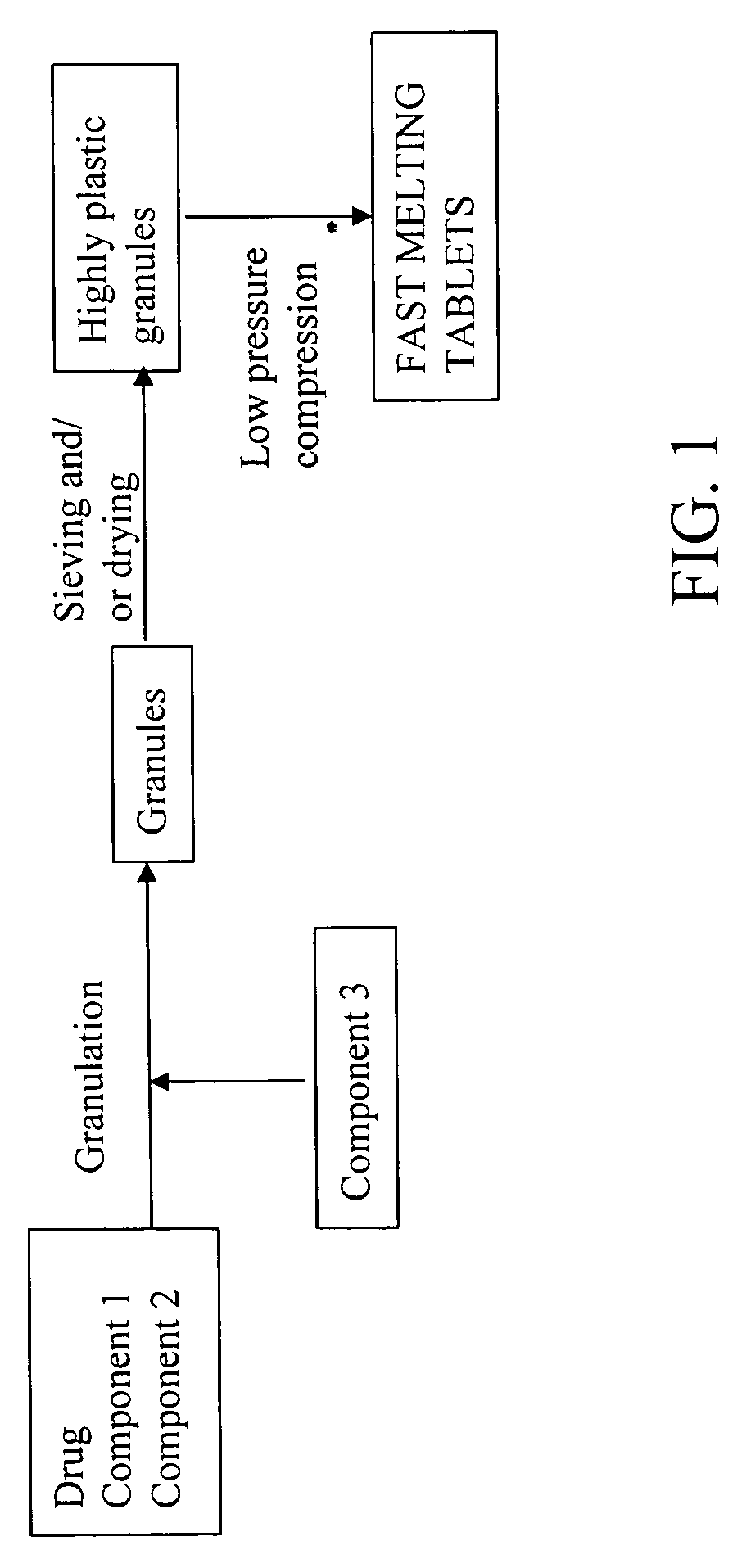
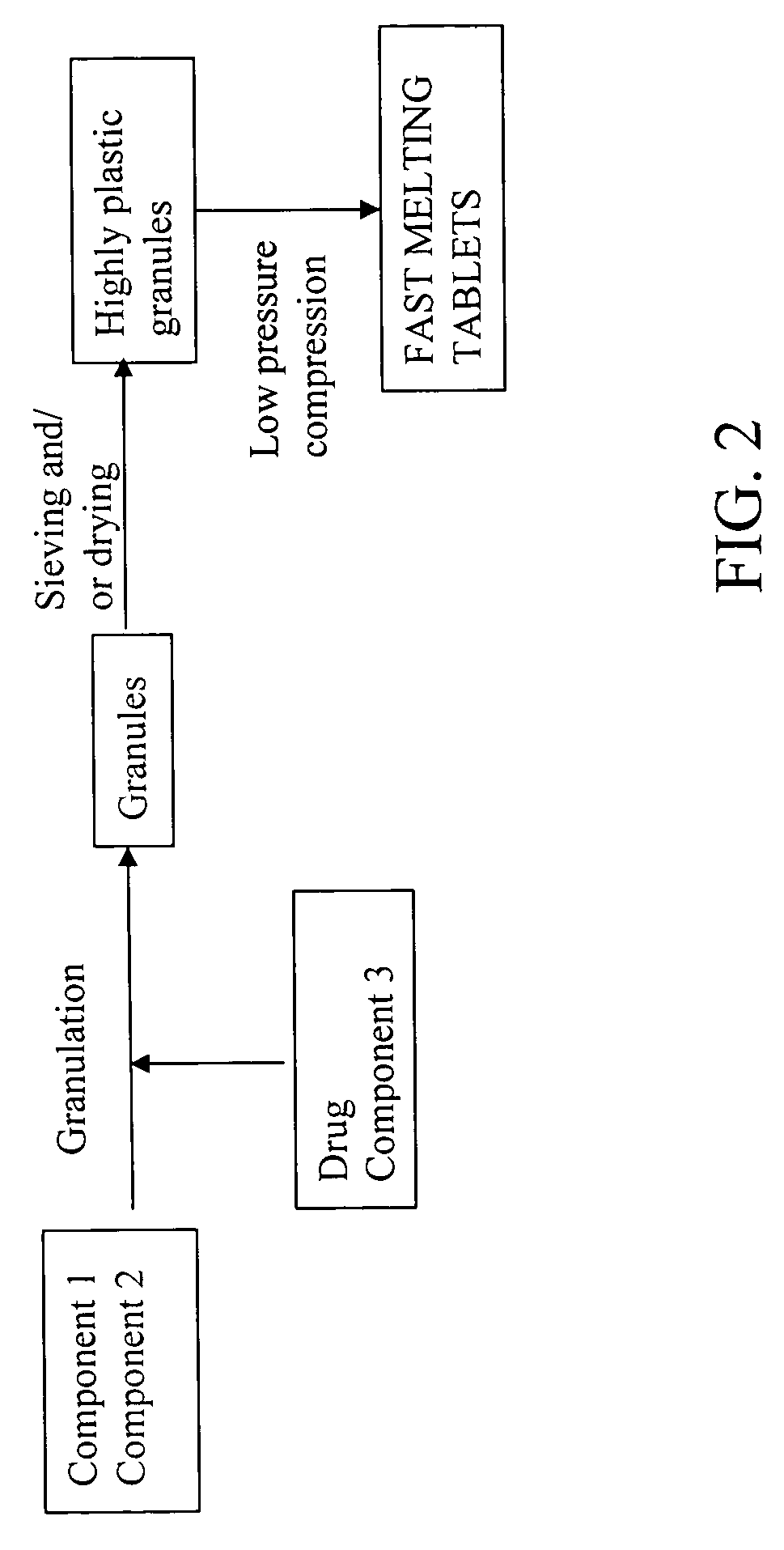
Highly plastic granules for making fast melting tablets
InactiveUS20050013857A1Increases tablet strengthIncrease tablet strengthPowder deliveryPill deliveryPlastic materialsHardness
A fast-melting pharmaceutical tablet comprises a porous, plastic substance, a water penetration enhancer and a binder. One or more drugs can be incorporated into the formulation at different stages of the process so as to afford a pharmaceutically active tablet. Methods of making the pharmaceutical tablet entail combining the porous, plastic material, the water penetration enhancing agent, and the binder so as to form highly plastic granules, which are compressed into tablets. The resulting tablets dissolve rapidly in the mouth and have good hardness with low brittleness. The tablets are particularly valuable to those who have difficulty swallowing conventional pills.
Owner:AKINA INC
Highly plastic granules for making fast melting tablets
A fast-melting pharmaceutical tablet comprises a porous, plastic substance, a water penetration enhancer and a binder. One or more drugs can be incorporated into the formulation at different stages of the process so as to afford a pharmaceutically active tablet. Methods of making the pharmaceutical tablet entail combining the porous, plastic material, the water penetration enhancing agent, and the binder so as to form highly plastic granules, which are compressed into tablets. The resulting tablets dissolve rapidly in the mouth and have good hardness with low brittleness. The tablets are particularly valuable to those who have difficulty swallowing conventional pills.
Owner:AKINA INC
Solid dispersing vaccine composition for oral delivery
InactiveUS20080014260A1Promote absorptionPotentiate immunogenic responseBacterial antigen ingredientsPharmaceutical delivery mechanismAdjuvantMicrosphere
The invention disclosed herein relate to an oral vaccine in which the vaccine composition and adjuvant(s) are carried on a solid fast-dispersing dosage form. The vaccines are targeted toward mucosal tissue and the adjuvant serves to ensure sufficient residence time for the vaccine composition on the mucosal tissue to facilitate its absorption thereby. The fast-dispersing oral solid vaccine dosage form of the invention is particularly useful to administer the vaccine to patients that have difficulty swallowing medications. In one embodiment, the invention provides a fast disintegrating oral solid vaccine dosage form comprising: an immunogenic amount of an antigenic preparation, the antigenic preparation comprising a microsphere-antigen complex; an adjuvant, wherein the adjuvant enhances the absorption of the antigen or potentiates the immunogenic response; a mucoadhesive substance; and a low density dosage form matrix.
Owner:SEAGER HARRY
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Lurasidone hydrochloride orally-disintegrating tablet preparation and preparation method thereof
InactiveCN103054824AOrganic active ingredientsNervous disorderLurasidone HydrochlorideOrally disintegrating tablet
The invention belongs to the technical field of medicine, and relates to a lurasidone hydrochloride orally-disintegrating tablet preparation and a preparation method thereof. The orally-disintegrating tablet comprises the following components in mass percent: 5-60 percent of lurasidone hydrochloride, 25-80 percent of filling material, 5-20 percent of disintegrating agent, 0.02-0.15 percent of wetting agent, 0.2-5 percent of flavoring agent and 0.5-2 percent of lubricating agent. The invention aims to provide a lurasidone hydrochloride orally-disintegrating tablet with simple preparation process, low cost, convenience in use and quick action to the indications; and compared with the conventional preparation for oral use, the lurasidone hydrochloride orally-disintegrating tablet preparation can reduce the difficulty in swallowing, improve the compliance, is suitable for special populations such as the elderly, children, patients with difficulty in swallowing and mental patients, can be fully disintegrated within 60 s, can be disintegrated into extremely fine powder, is higher in drug dissolution rate and quick in absorption, and can improve the bioavailability of insoluble drugs, for example, lurasidone hydrochloride.
Owner:BEIJING VENTUREPHARM BIOTECH
Solid composition comprising a proton pump inhibitor
The present invention related to a method for oral administration of a solid composition comprising an acid labile proton pump inhibitor compound in the form of a multiple of enteric coating layered pellets, wherein the pellets are in admixture with one or more pharmaceutically acceptable thickeners capable of forming a viscous medium when dispersed in an aqueous carrier. Alternatively, the enteric coated pellets are in admixture with a viscous medium. The formed aqueous viscous suspension is administered through a gastric tube. The method and composition are especially aimed for treatment of patients in need of a proton pump inhibitor and having difficulties to swallow or for pediatric patients.
Owner:ASTRAZENECA AB
Method for administering medicaments to subjects with swallowing difficulties and disorders
InactiveUS20070196495A1Easy to swallowPowder deliveryHeavy metal active ingredientsOral medicationPharmaceutical drug
The present invention is a variably thickened pharmaceutical dosage form, its composition and its use for orally administering medications to patients that have difficulty swallowing other solid dosage forms such as tablets or capsules.
Owner:ALTE BIOSCI
Pharmaceutical composition of topiramate
The invention is directed to a pharmaceutical composition of topiramate, an anticonvulsant which is useful for treating epilepsy. More specifically, the present invention provides a solid dosage formulation of topiramate intended primarily for use by pediatric patients, or for patients who have difficulty swallowing tablets. Processes for preparing the pharmaceutical composition are also described.
Owner:ORTHO MCNEIL PHARM INC
Temperature sensitive gel sustained-release oral agent with paracetamol
InactiveCN102600066AWide variety of sourcesEasy to getOrganic active ingredientsAntipyreticPolysaccharide formationBULK ACTIVE INGREDIENT
The invention discloses a temperature sensitive gel sustained-release oral agent with a paracetamol, and relates to the technical field of pharmaceutic preparation. A carrier in the oral agent provided by the invention convenient for feeding includes paracetamol serving as an active ingredient and xyloglucan or a mixture of xyloglucan and another polysaccharide, wherein another polysaccharide is pectin, chitosan, algal polysaccharides or gellan gum. According to the invention, a polymer three-dimensional gel net structure formed by the polysaccharide is used for establishing a sustained-release system combined with clinical pathology so as to obtain the temperature sensitive gel sustained-release oral agent with the paracetamol; the temperature range for sol-gel change is 35-37 DEG C which is close to body temperature, so the oral agent is particularly suitable for old people, infants and people who have difficulty in swallowing, and has obvious efficacy. The temperature sensitive gel sustained-release oral agent disclosed by the invention has obvious analgesic and antipyretic effects, is convenient for administration and free of toxic and side effects, and the drug action can last for a long time.
Owner:SHANDONG XIFENGTIAN ECOLOGY FERTILIZER IND
Procaterol hydrochloride granules and preparation process thereof
InactiveCN105232470AImprove solubilityPromote meltingOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseAsthmatic bronchitis
The invention relates to procaterol hydrochloride pharmaceutical granules, which have an effect of relieving asthma, and a preparation process of the granules. The procaterol hydrochloride granules belong to an orally rapidly dispersible preparation which is prepared from procaterol hydrochloride and medicinal pharmaceutical excipients, and the preparation is suitable for treating bronchial asthma, asthmatic bronchitis and chronic obstructive pulmonary disease. According to the invention, the prepared granule medicine is rapid to be dissolved and uniform to be dispersed; the medicine can be taken after being dissolved in water and the medicine can be directly taken without water as well; the medicine is delicate and sweet in taste; the medicine is capable of effectively overcoming medication rejecting psychology of patients and meeting a medication requirement during an asthma acute attacking period, and the medicine is especially suitable for children, old people or patients having difficulty in swallowing. Upon detection, the procaterol hydrochloride granules prepared by the invention are quite excellent in static dissolubility, and are obviously better than a preparation of a conventional process in content uniformity; and the procaterol hydrochloride granules effectively guarantee the timeliness, the safety and the effectiveness of clinical treatment, and the procaterol hydrochloride granules are quite high in value of popularization.
Owner:HEILONGJIANG LONGDE PHARMA CO LTD
Pharmaceutical composition of topiramate
The invention is directed to a pharmaceutical composition of topiramate, an anticonvulsant which is useful for treating epilepsy. More specifically, the present invention provides a solid dosage formulation of topiramate intended primarily for use by pediatric patients, or for patients who have difficulty swallowing tablets. Processes for preparing the pharmaceutical composition are also described.
Owner:THAKUR MADHAV S +2
Pyridostigmine bromide odor masking orally disintegrating tablets and preparation method thereof
ActiveCN102309460AGreat tasteTaste Improves the taste of the drug while improvingOrganic active ingredientsMuscular disorderPyridostigmine BromideOrally disintegrating tablet
The invention relates to pyridostigmine bromide odor masking orally disintegrating tablets and a preparation method thereof. The tablets belong to orally disintegrating tablets, and the orally disintegrating tablets consist of pyridostigmine bromide, an odor masking agent, a filling agent, a disintegrating agent and a flavoring agent basically, can be prepared by a direct tablet compressing process or a wet granulation process, have good mouthfeel and are disintegrated for 20 to 45 seconds; and in the dissolution detection process, the average cumulative dissolution quantity in 0.1mol / L of hydrochloric acid medium within 5 minutes is more than 85 percent. The tablets have good mouthfeel and quick response, are quickly disintegrated and convenient to take, and are particularly suitable to be taken by the old, children and patients who suffer from dysphagia and are in special environments (such as an environment in which water is difficult to take). The preparation method does not have special requirements on equipment, and products have controllable quality and low cost, and are easy to produce industrially.
Owner:CHONGQING MEDICAL UNIVERSITY
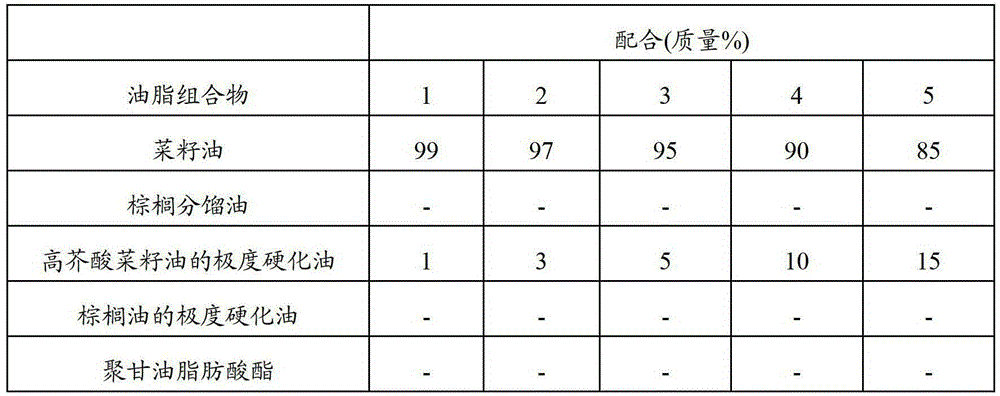
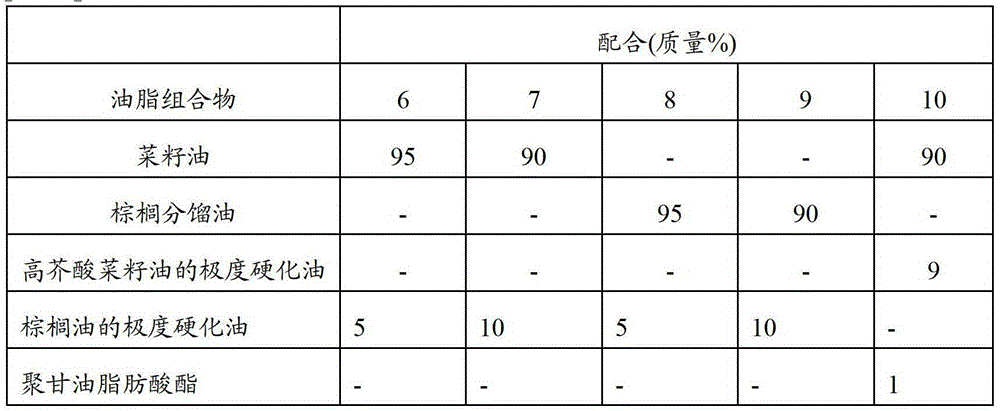
Ingestion-assisting oleaginous composition for persons having difficulty swallowing/masticating, and food for persons having difficulty swallowing/masticating
InactiveCN103096725AAvoid attenuationEasy to cleanAnimal feeding stuffAccessory food factorsFood itemFood science
The present invention relates to: an ingestion-assisting oleaginous composition that is for persons having difficulty swallowing / masticating and that is obtained by rapidly cooled mixing / kneading processing of a composition having an SFC of 3-18% in the range of 5-25 DEG C and containing an oil and an emulsifier having an HLB value of 1-10; a food for persons having difficulty swallowing / masticating containing shredded or pulverized edible food and the ingestion-assisting oleaginous composition for persons having difficulty swallowing / masticating; and a method that is for producing the food for persons having difficulty swallowing / masticating and that is characterized by mixing shredded or pulverized edible food with the ingestion-assisting oleaginous composition that is for persons having difficulty swallowing / masticating and that is obtained by rapidly cooled mixing / kneading processing of a composition having an SFC of 3-18% in the range of 5-25 DEG C and containing an oil and an emulsifier having an HLB value of 1-10. As a result, it is possible to provide: an ingestion-assisting oleaginous composition for persons having difficulty swallowing / masticating that can easily produce a food that persons having difficulty swallowing / masticating can easily consume; and a food for persons having difficulty swallowing / masticating that uses same.
Owner:THE NISSHIN OILLIO GRP LTD
Olanzapine oral instant film
ActiveCN104546807BHigh drug loadingThe overall thickness is thinOrganic active ingredientsNervous disorderPharmaceutical medicineDrug loading dose
The invention relates to an olanzapine oral instant film which can be dissolved immediately in the oral cavity, which is used for improving the use performance of the olanzapine and belongs to the field of pharmaceutical preparations. The mouth-dissolving film includes active pharmaceutical ingredients and pharmaceutically applicable auxiliary materials, the active pharmaceutical ingredient is olanzapine, and pharmaceutically applicable auxiliary materials include: film-forming materials, plasticizers, absorption accelerators, flavoring agents and other auxiliary materials, film-forming materials Include at least gelatin. This orally dissolving film has the advantages of high drug loading, thin thickness, good taste, instant dissolution in the oral cavity without drinking water, and fast oral absorption, which solves the poor drug compliance of schizophrenic patients and the phenomenon of Tibetan medicine and vomiting. Especially suitable for patients with swallowing difficulties.
Owner:QILU PHARMA
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Compositions and methods for improving hydration of individuals having dysphagia
InactiveCN109069453AEasy to drinkPromote hydrationFood ingredient as thickening agentDispersion deliveryPharmacologyDehydration
A method of treating or preventing dehydration in an individual having impaired swallowing such as dysphagia can include administering to the individual having the dysphagia an effective amount of a composition containing a salivating agent and a cooling agent in a weight ratio of 1:0.06 to 1:0.2. Optionally the composition includes a tingling agent. The composition can be a powder that is reconstituted before administration, for example at the point of consumption. A thickening or thin cohesive agent can be included in the powder and / or can be included in a liquid in which the powder is reconstituted. The composition can be a beverage containing the salivating agent and the cooling agent in a total amount of at least 3.0 wt.% of the beverage, for example at least 6.0 wt.% of the beverage.The composition can be a ready-to-drink beverage or a frozen dessert on a stick.
Owner:SOC DES PROD NESTLE SA
Orally Disintegrating Tablet Compositions of Lamotrigine
The compositions of the present invention composition comprise a therapeutically effective amount of particles comprising lamotrigine, in combination with granules comprising a disintegrant, and a sugar alcohol and / or a saccharide. These compositions are useful in treating epilepsy and bipolar disorder, particularly for patients with dysphagia, and to improve compliance with bipolar patients.
Owner:ADARE PHARM INC
Pharmaceutical composition formulated for pre-gastric absorption of monoamine oxidase B inhibitors
InactiveUS20050106241A1Avoid disadvantagesImproves ease and convenienceBiocideOrganic active ingredientsGastric AbsorptionMetabolite
The invention described herein provides a fast dispersing oral solid dosage form containing monoamine oxidase B inhibitor (MAO-B inhibitor) as the active ingredient, and method of treating disease therewith, such as Parkinson's disease. In one embodiment, the monoamine oxidase B inhibitor selegiline or its analogue can be the sole active ingredient in the composition administered. The dosage form composition is formulated to promote absorption through the buccal, sublingual, pharyngeal and / or esophageal mucous membrane tissue, such that at least 5% of the active ingredient is absorbed within one minute of placement in the oral cavity, as determined by a buccal absorption test. Monoamine oxidase B inhibitor compounds administered in accordance with the invention decrease the amount of undesirable metabolites associated with first pass effect of selegiline, for example, such as amphetamine and methamphetamine. The invention provides a number of other advantages over conventional orally administered tablet forms, including administration of monoamine oxidase B inhibitor compounds to patients that have difficulty swallowing.
Owner:CATALENT USA WOODSTOCK INC +2
Effervesce tablet of American ginseng containing vitamin C and preparation method thereof
InactiveCN100508955CEasy to carryKeep the essenceOrganic active ingredientsMetabolism disorderEffervescent tabletVitamin C
The invention discloses an American ginseng effervescent tablet containing vitamin C and a preparation method thereof. The invented product is easy to carry and can be taken by effervescence anytime and anywhere. It retains the ginseng essence of the extract. After effervescence, the fragrance of the original ginseng itself is restored. It provides convenience for people who have difficulty swallowing, without going through the process of gastrointestinal disintegration.
Owner:谢谦谦
Fat and oil composition for ingestion aid of food for persons with difficulty in swallowing and chewing, and food for persons with difficulty in swallowing and chewing
InactiveCN103096725BAvoid attenuationEasy to cleanAccessory food factorsFood coatingFat compositionCHEWING DIFFICULTY
The present invention relates to an oil and fat composition for feeding aids for people with difficulty in swallowing and chewing. Its SFC in the range of 5 to 25°C is 3 to 18%, and it is obtained by emulsifying the oil containing oil and having an HLB value of 1 to 10 The composition obtained by quenching and kneading the composition of the preparation; the food for people with swallowing and chewing difficulties, which contains the above-mentioned oil and fat composition for feeding aids for people with swallowing and chewing difficulties and minced food that can be ingested or Pulverized product; a method for producing a food for people with swallowing and chewing difficulties, wherein the oil and fat composition for feeding aids for people with swallowing and chewing difficulties is mixed with chopped or pulverized foods that can be ingested. The oil and fat composition for feeding aids for difficult persons has an SFC of 3 to 18% in the range of 5 to 25°C, and is obtained by quenching and kneading a composition containing fat and oil and an emulsifier with an HLB value of 1 to 10 of. According to the present invention, it is possible to provide an oil and fat composition for assisting feeding for people who have difficulty swallowing and chewing, and a food for people who have difficulty swallowing and masticating using the oil and fat composition, wherein the oil and fat composition can be easily produced and swallowed, Foods that are easy to ingest for those who have difficulty chewing.
Owner:THE NISSHIN OILLIO GRP LTD
Capsule dosage form of metoprolol succinate
ActiveUS9700530B2Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
Owner:SUN PHARMA INDS
Solid dispersing vaccine composition for oral delivery
The invention disclosed herein relate to an oral vaccine in which the vaccine composition and adjuvant(s) are carried on a solid fast-dispersing dosage form. The vaccines are targeted toward mucosal tissue and the adjuvant serves to ensure sufficient residence time for the vaccine composition on the mucosal tissue to facilitate its absorption thereby. The fast-dispersing oral solid vaccine dosage form of the invention is particularly useful to administer the vaccine to patients that have difficulty swallowing medications. In one embodiment, the invention provides a fast disintegrating oral solid vaccine dosage form comprising: an immunogenic amount of an antigenic preparation, the antigenic preparation comprising a microsphere-antigen complex; an adjuvant, wherein the adjuvant enhances the absorption of the antigen or potentiates the immunogenic response; a mucoadhesive substance; and a low density dosage form matrix.
Owner:SEAGER HARRY
Capsule dosage form of metoprolol succinate
ActiveUS20170042837A1Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
This disclosure provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units, wherein said capsule dosage form is bioequivalent to the marketed ToprolXL® tablet. The extended-release capsule dosage form comprising coated discrete units can be sprinkled onto food to ease administration to patients who have difficulty swallowing tablets or capsules.
Owner:SUN PHARMA INDS
Compositions and methods for improving hydration in individuals with dysphagia
PendingCN114470066APromote hydrationReduce mortalityFood ingredient as thickening agentDispersion deliverySwallowing impairmentPharmacology
Owner:SOC DES PROD NESTLE SA
Ginseng freeze-dried orally disintegrating tablet and preparation method thereof
ActiveCN110787201BNervous disorderMetabolism disorderOrally disintegrating tabletPharmaceutical drug
The invention relates to the fields of medicine and food, in particular to a ginseng freeze-dried orally disintegrating tablet and a preparation method thereof. The present invention solves the rapid disintegration of tablets in the oral cavity through freeze-drying technology in ginseng medicine, food and health food. Good, suitable for people who have difficulty swallowing, such as the elderly and children, to improve medication compliance, and suitable for people with inconvenient drinking water.
Owner:辽宁祥云健康产业股份有限公司
Dysphagia cup
A cup for a patient suffering from dysphagia comprises a base upon which the cup is capable of standing when on a level surface and a leading wall extending from the base. The leading wall has a first portion that is substantially planar or concave when viewed from outside the cup, the first portion being at an angle of at least 100 degrees relative to the base. The cup also comprises a trailing wall opposite the leading wall, wherein the trailing wall extends from the base to the lip of the cup; and side walls extending between the leading and trailing walls, the side walls being convex when viewed from outside of the cup. The lip of the cup is upwardly inclined relative to the base in the direction of the trailing wall to the leading wall. The cup allows a patient suffering from dysphagia to drink without having to unduly tip back his or her head.
Owner:FLAVOUR CREATIONS PTY LTD
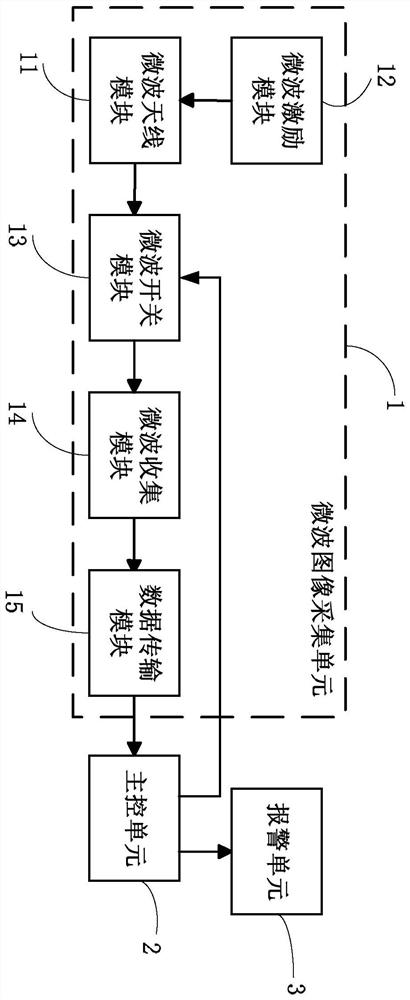
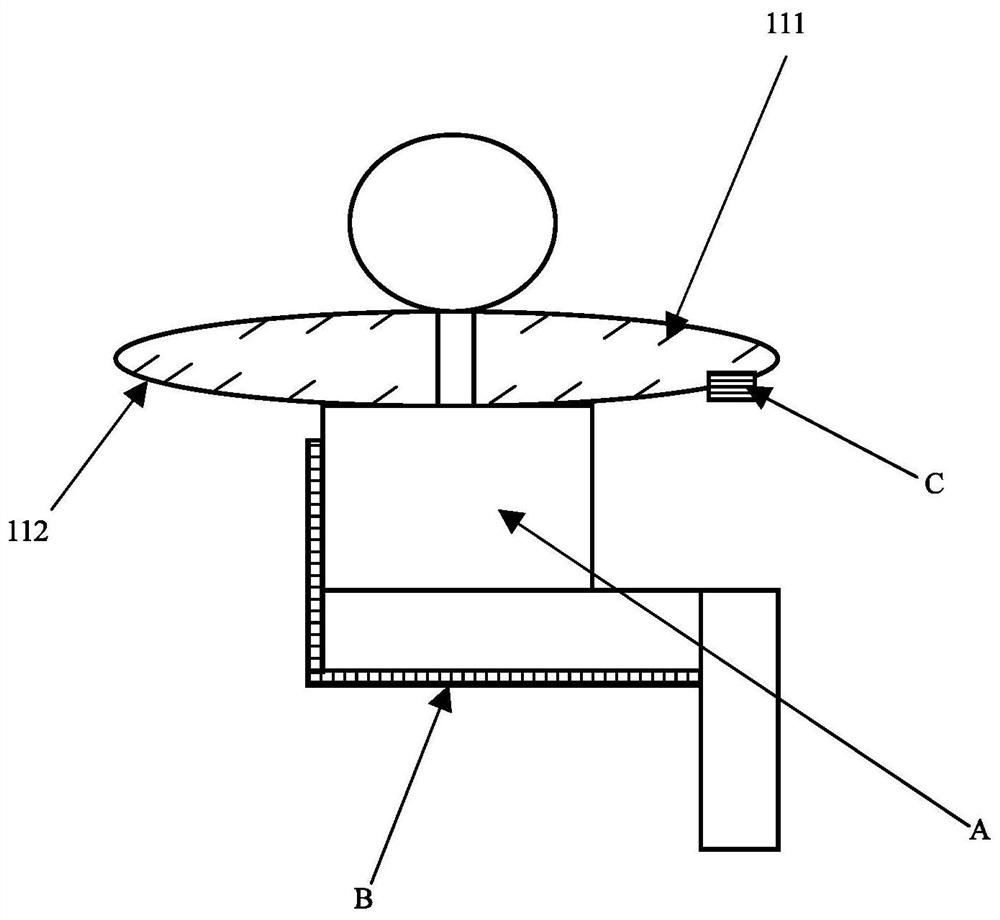
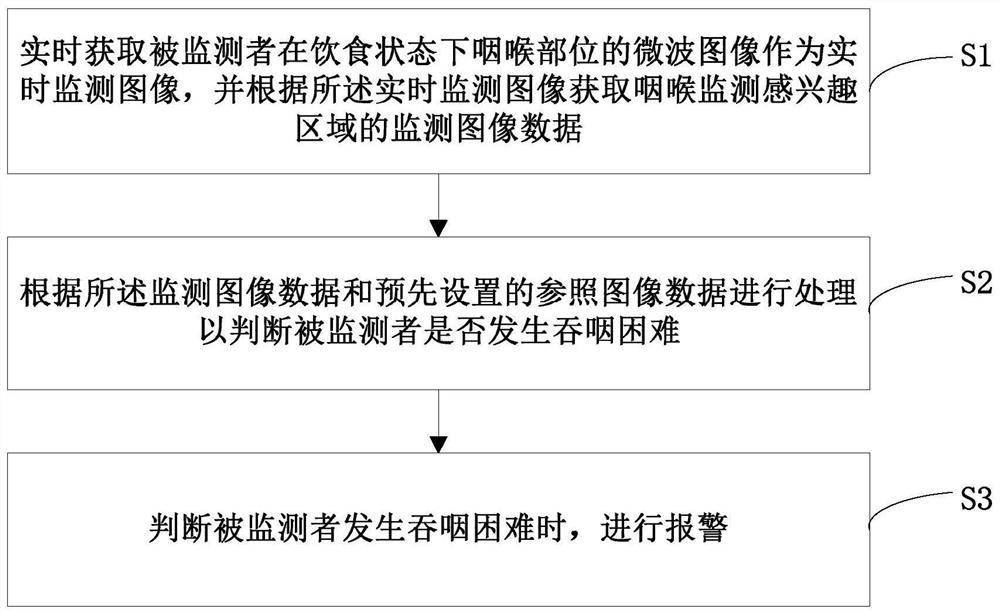
A swallowing real-time monitoring method and system thereof
ActiveCN108652621BMonitor for dysphagiaEffective monitoring of dysphagiaDiagnostic recording/measuringSensorsDifficult swallowingPhysical medicine and rehabilitation
The invention discloses a swallowing real-time monitoring method and system thereof. The microwave image acquisition unit acquires the microwave image of the throat of the monitored person in a state of eating and drinking in real time as a real-time monitoring image, and the main control unit performs processing according to the real-time monitoring image and reference image data. To determine whether the monitored person has dysphagia. It overcomes the technical problems of poor real-time swallowing monitoring in the existing technology, poor experience of the monitored person, and difficult access to professional inspection equipment or drugs, and realizes real-time and effective monitoring of whether the monitored person has dysphagia, thereby improving the quality of the monitored person. quality of life of the patient.
Owner:YITI INTELLIGENT TECH LTD SHENZHEN CITY
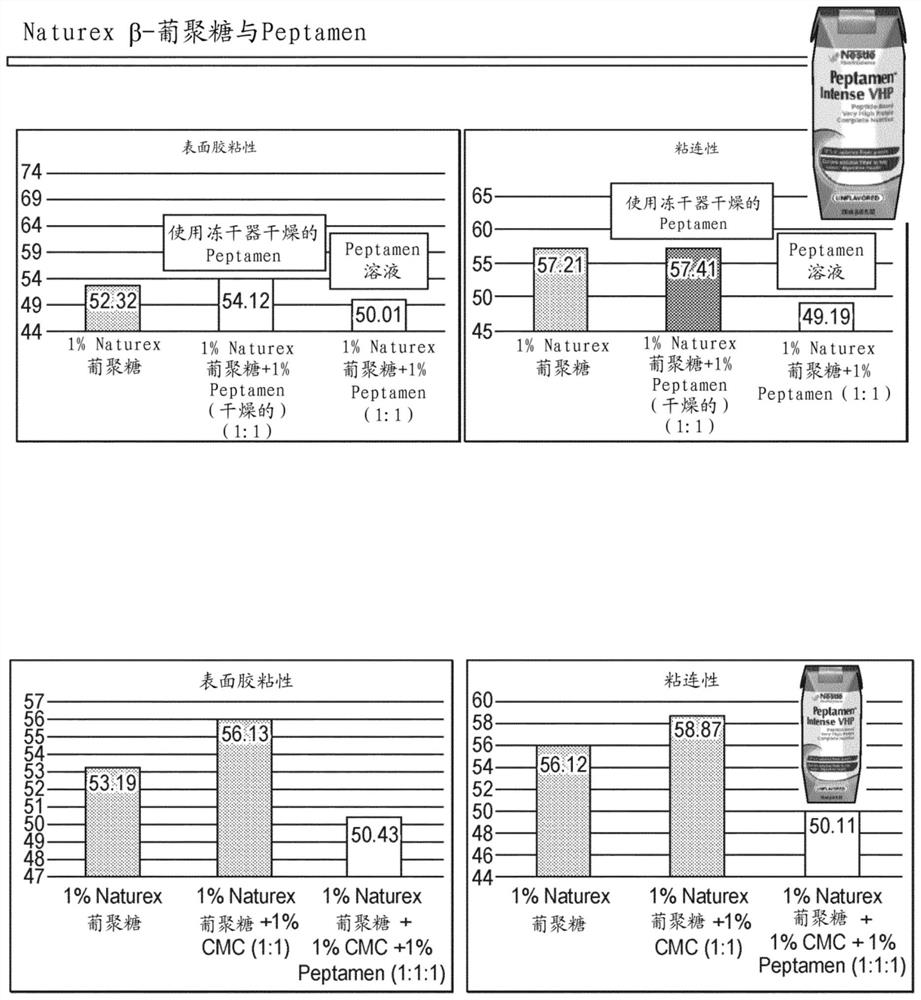
Thickening agents and nutritional products to promote safe swallowing of individuals with dysphagia and methods of making and using same
PendingCN114258269AReduce flowReduce cohesionOrganic active ingredientsDigestive systemPhysical medicine and rehabilitationNutrition
A thickener, as well as nutritional products comprising the thickener, uses thereof, methods of manufacture thereof, methods for improving cohesiveness of nutritional products, and related systems are disclosed. The nutritional product has improved cohesiveness to promote a safer and more efficient swallowing of food masses by individuals with dysphagia, such as dysphagia. In a preferred embodiment, the nutritional product comprises a thickening agent, the thickening agent comprising beta-glucan and an additive. Preferably, the nutritional product has a relaxation time of from 10 ms to 2000 ms as measured by the CaBER experiment at a temperature of 20 DEG C.
Owner:SOC DES PROD NESTLE SA
Powdered thickener maintaining its extensional properties when reconstituted and for promoting safe swallowing by individuals with dysphagia
PendingCN113163831ASafe to swallowIncrease shear viscosityFood ingredient as thickening agentDigestive systemNutritionGlucan
A thickening powder promotes safe swallowing of a nutritional product by an individual with dysphagia and can be used in methods of treating dysphagia, promoting safe swallowing of a nutritional product, and mitigating a risk of aspiration during swallowing of a nutritional product. The powder contains beta-glucan and at least one carrier ingredient in an amount that is neutral toward or enhances the extensional properties of the nutritional product. The at least one carrier ingredient can be one or more of maltodextrin, isomaltulose, sucrose or lactose. The powder can be diluted in a diluent to form an aqueous solution that is at least a portion of a nutritional product and to improve the cohesiveness of the nutritional product. Preferably, the resultant nutritional product has a shear viscosity greater than 200 mPas and up to about 2,000 mPas, preferably greater than 200 mPas and up to about 500 mPas, more preferably 250 mPas to about 450 mPas, most preferably 250 mPas to about 400 mPas, when measured at a shear rate of 50s-1 at a temperature of 20 DEG C.
Owner:SOC DES PROD NESTLE SA
Capsule dosage form of metoprolol succinate
InactiveUS20170189351A1Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
This disclosure provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units, wherein said capsule dosage form is bioequivalent to the marketed Toprol-XL® tablet. The extended-release capsule dosage form comprising coated discrete units can be sprinkled onto food to ease administration to patients who have difficulty swallowing tablets or capsules.
Owner:SUN PHARMA INDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com