Process for preparing cross-linked sodium hyaluronate microspheres capable of being adopted as emboliaztion agent by adopting sodium hyaluronate as raw material
A cross-linked hyaluronic acid and sodium hyaluronate technology, which is applied in the field of interventional medicine, can solve the problems of cross-linked hyaluronic acid microbeads without effective oil phase residue, cross-linking agent residue control measures, and inapplicability. Good biocompatibility and degradability, good microsphere shape, and easy sieving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
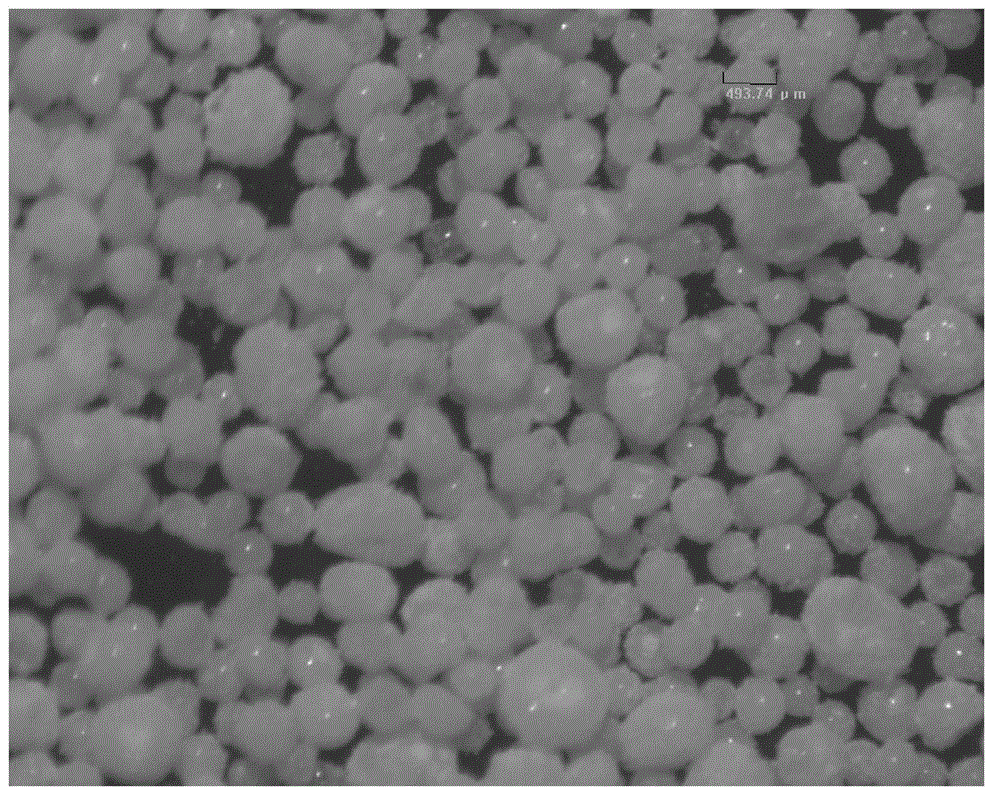
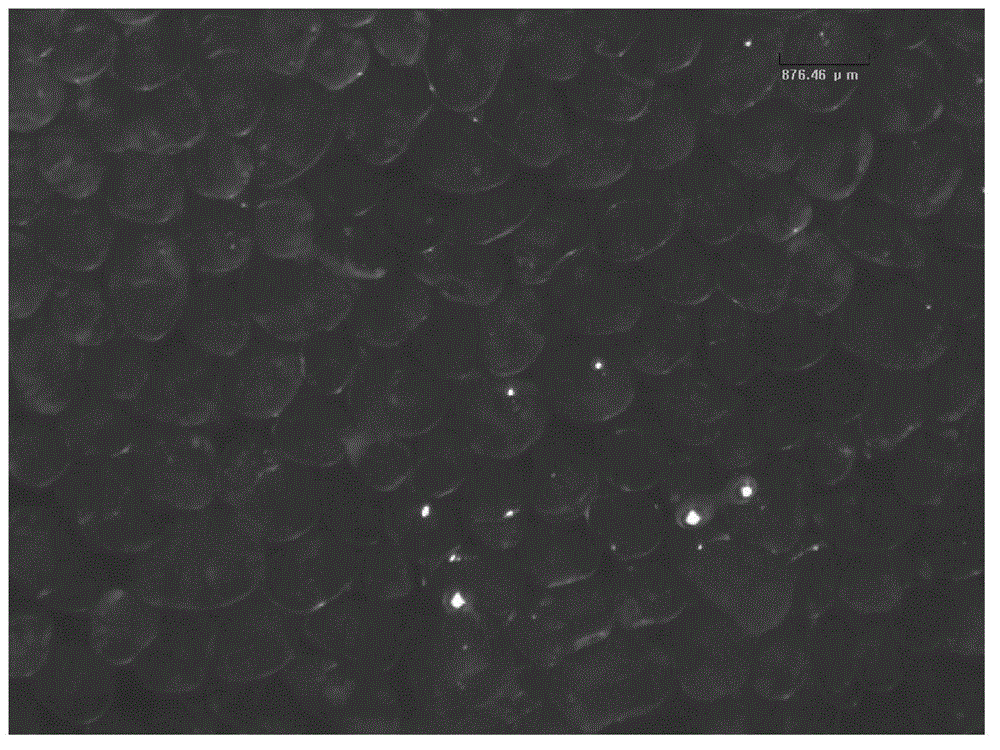
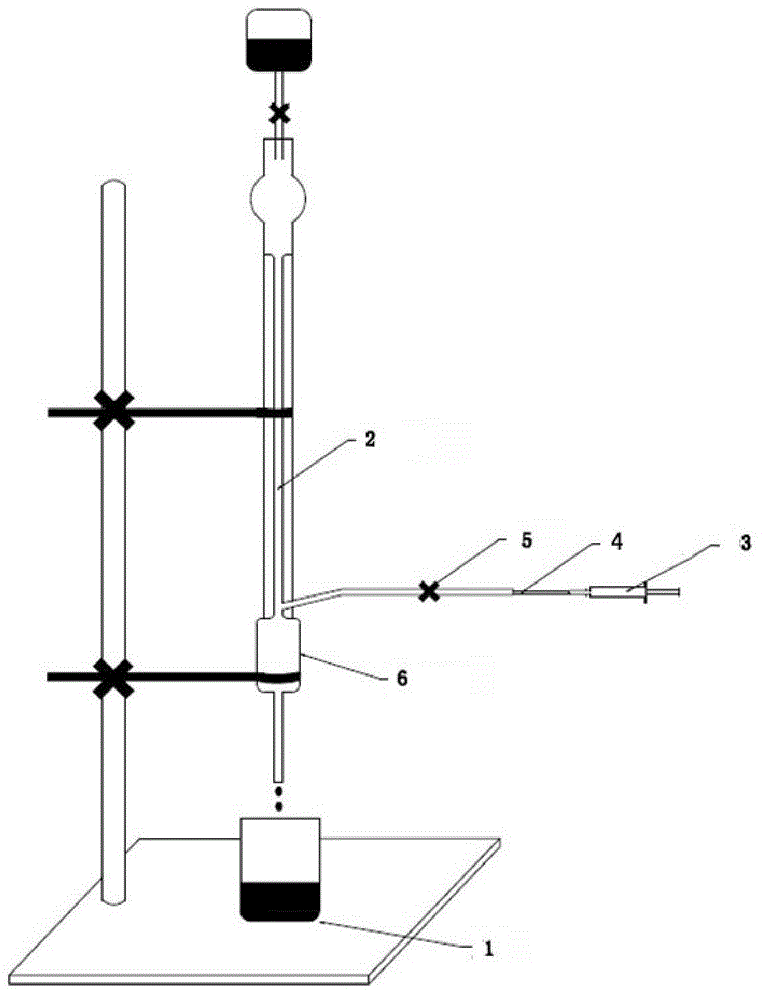
Image
Examples
Embodiment 1
[0028] (1) Weigh 3.07 g of sodium hyaluronate dry powder with a molecular weight of 1.3 million Daltons, and prepare it with 1% NaOH solution to form a sodium hyaluronate lye gel with a mass percent concentration of 10% g / ml;
[0029] (2) Mix 100.05g of liquid paraffin and 2.03g of Span80 to obtain the oil phase, slowly add the sodium hyaluronate gel in step (1) into the oil phase, emulsify at high speed with a mixer, the emulsification speed is 1000rpm, and the time is 10min;
[0030] (3) After the emulsification in step (2) is completed, add 1ml of cross-linking agent (BDDE), stir at room temperature for 4 hours for cross-linking reaction, and let stand overnight after completion;
[0031] (4) Pour off the upper oil phase, and repeatedly wash the remaining oil phase on the surface of the microspheres with ethyl acetate, and then repeatedly wash the microspheres with absolute ethanol to remove ethyl acetate;
[0032] (5) Put the microspheres obtained in step (4) into a vacuu...
Embodiment 2
[0037] (1) Weigh 3.02 g of sodium hyaluronate dry powder with a molecular weight of 1.3 million Daltons, and mix it with 1% NaOH to prepare a sodium hyaluronate lye gel with a mass percent concentration of 15% g / ml;
[0038] (2) Mix 100.12g of liquid paraffin and 2.04g of Span80 to obtain an oil phase, slowly add the sodium hyaluronate gel in step (1) into the oil phase, and emulsify with high-speed shearing by an emulsifier at a shear rate of 1000rpm, the time is 10min;
[0039] (3) After the emulsification in step (2) is completed, add 1ml of cross-linking agent (BDDE), stir at room temperature for 4 hours for cross-linking reaction, and let stand overnight after completion;
[0040] (4) Pour off the upper oil phase, and repeatedly wash the remaining oil phase on the surface of the microspheres with ethyl acetate, and then repeatedly wash the microspheres with absolute ethanol to remove ethyl acetate;
[0041] (5) Put the microspheres obtained in step (4) into a vacuum dryi...
Embodiment 3
[0048] (1) Weigh 3.03 g of sodium hyaluronate dry powder with a molecular weight of 1.3 million Daltons, and mix it with 1% NaOH to prepare a sodium hyaluronate lye gel with a mass percent concentration of 10% g / ml;
[0049] (2) Mix 150.15g of liquid paraffin and 2.68g of Span80 to obtain an oil phase, slowly add the sodium hyaluronate gel in step (1) into the oil phase, and emulsify with high-speed shearing by an emulsifier at a shear rate of 1000rpm, the time is 10min;
[0050] (3) After the emulsification in step (2) is completed, add 1ml of cross-linking agent (BDDE), stir at room temperature for 4 hours for cross-linking reaction, and let stand overnight after completion;
[0051] (4) Pour off the upper oil phase, and repeatedly wash the remaining oil phase on the surface of the microspheres with ethyl acetate, and then repeatedly wash the microspheres with absolute ethanol to remove ethyl acetate;
[0052] (5) Put the microspheres obtained in step (4) into a vacuum dryi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com