Mesenchymal stem cell low-temperature preserving fluid and preparation method thereof
A technology of mesenchymal stem cells and cryopreservation, applied in the field of stem cell preservation, can solve the problems of inability to use, decreased cell viability, and cannot be used as a preservation solution for mesenchymal stem cells for clinical treatment, etc. Avoid the effects of the freeze-thaw step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
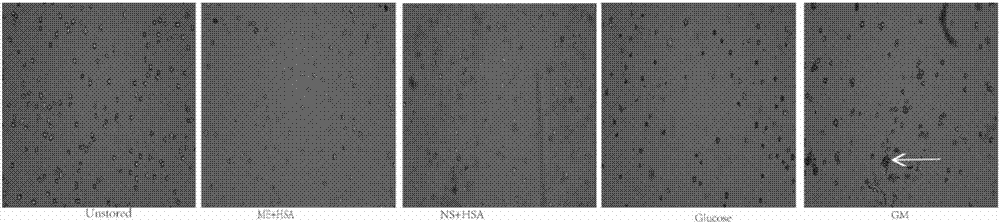
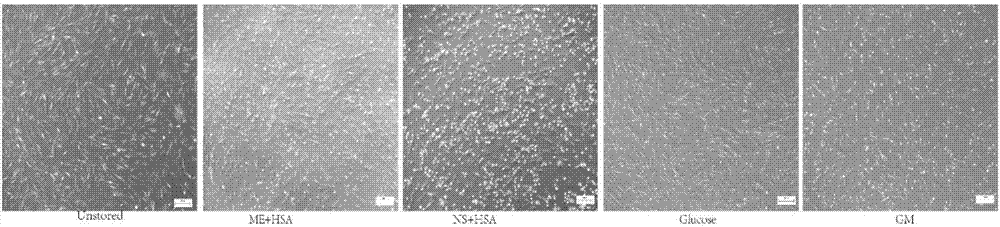
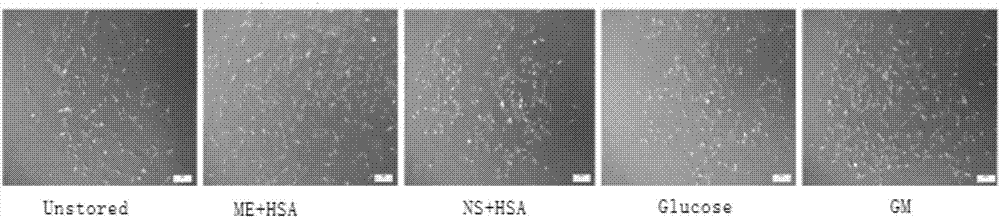
[0028] Example 1: Comparison of cell viability, adherence and clone formation between the mesenchymal stem cell injection prepared from a mesenchymal stem cell cryopreservation solution of the present invention and injections prepared from other formulations
[0029] 1.1 Preparation of mesenchymal stem cell injection of the present invention and other formula injections
[0030] The formulations, storage temperature and storage time of different storage solutions are shown in Table 1.
[0031] The Unstored group is the control group, that is, 10% fetal bovine serum (FBS) is added to α-MEM to prepare a preservation solution, and then the freshly digested cells are suspended in the preservation solution to a cell density of 1×106 cells / ml, without preservation operation, directly to the experiment.
[0032] The compound electrolyte injection (ME) + human serum albumin (HSA) group is the mesenchymal stem cell injection of the present invention, that is, 5% HSA is added to the co...
Embodiment 2
[0053] Example 2: The mesenchymal stem cell injection prepared from a mesenchymal stem cell cryopreservation solution of the present invention was tested for proliferation ability, cell osteogenic differentiation ability test, cell adipogenic differentiation ability test and cell surface marker detection.
[0054] 2.1 Preparation of mesenchymal stem cell injection of the present invention
[0055] Add 5% HSA to the compound electrolyte injection to prepare a preservation solution, and then suspend freshly digested cells in the preservation solution to a cell density of 5×10 6 per ml, stored for 24 hours before experimenting.
[0056] The Unstored group is the above-mentioned Unstored group, which is the fresh cells digested after the cell preparation, and does not undergo cryopreservation. The Unstored group is the control group, which symbolizes the best state of the cells. This group is set to test whether the state of the cells changes significantly after our cryopreserva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com