Pharmaceutical composition prepared by combining paclitaxel with CDKS kinase inhibitor for use
A kinase inhibitor and paclitaxel technology, applied in the field of chemical medicine, can solve the problem of ineffectiveness of anticancer agents, and achieve the effects of reducing clinical dosage, good anticancer efficacy, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
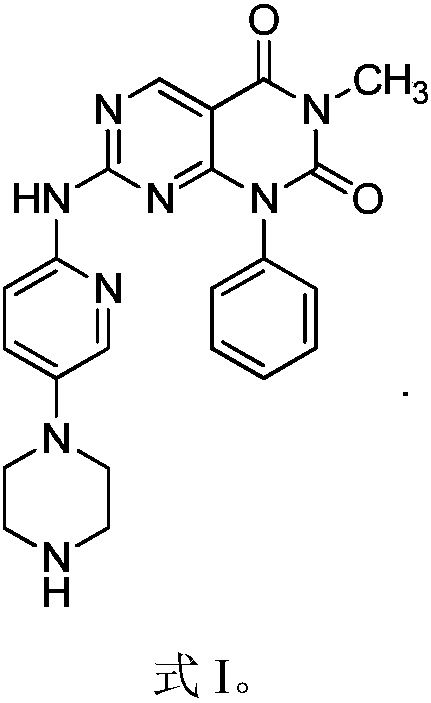
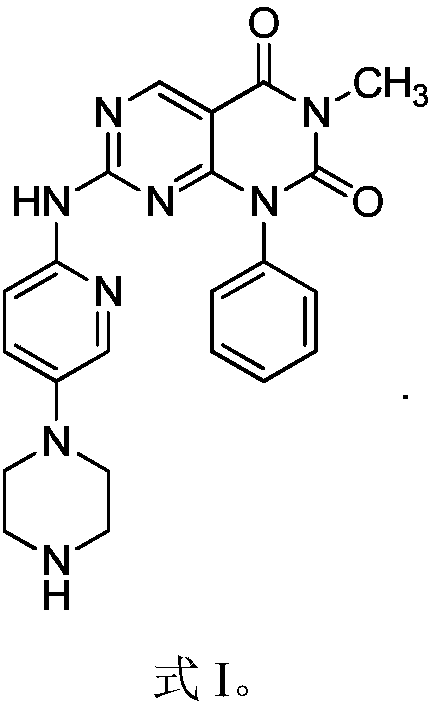
[0018] Example 1 1-Phenyl-3-methyl-7-(5-piperazinylpyridin-2-ylamino)pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione Synthesis
[0019]
[0020] Step 1 Synthesis of 1-tert-Butoxycarbonyl-4-(2-aminopyridin-5-yl)piperazine
[0021]
[0022] Weigh 3.15g of anhydrous piperazine in the reaction flask, add 30m dichloromethane to dissolve, slowly add dropwise to dissolve 4g (BOC) at 0℃ 2 Dichloromethane solution of O, after dropping, react at room temperature for 2h, filter, evaporate the solvent, add 10ml of water, filter, add sodium carbonate to the aqueous solution to saturation, extract with dichloromethane 3 times, collect the organic phase, dry with sodium sulfate, filter, Spin to dryness to obtain 2.98 g of oil. Add 50ml of DMF / water mixed solvent with a volume ratio of 1:1 to dissolve the obtained oil, add 0.41g of 2-amino-5-chloropyridine, and react at 100°C for 8 hours. After the reaction is over, add 20ml of water, extract with ethyl acetate, and anhydrous sulfuric acid The organi...
Embodiment 2
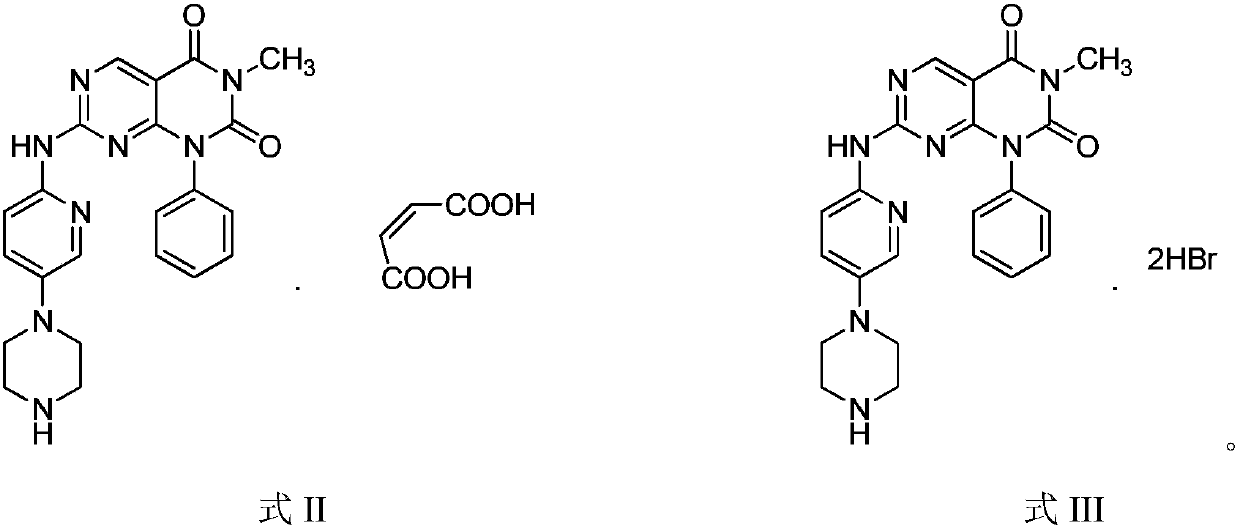
[0048] Example 2 1-Phenyl-3-methyl-7-(5-piperazinylpyridin-2-ylamino)pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione Synthesis of maleate
[0049]
[0050] Weigh 10.78g of the compound of Example 1 in a reaction flask, add 50ml of methanol aqueous solution with a volume ratio of 2:1 to dissolve, add 2.9g of maleic acid, heat to 45°C and stir for 0.5h, cool to room temperature, and cool in the refrigerator overnight , Filter, wash with methanol water with a volume ratio of 2:1, and dry to obtain the title compound.
Embodiment 3
[0051] Example 3 1-Phenyl-3-methyl-7-(5-piperazinylpyridin-2-ylamino)pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione Synthesis of hydrobromide
[0052]
[0053] Weigh 15g of the compound of Example 1 in a reaction flask, add 50ml of dichloromethane to dissolve, add 5.9g of hydrobromic acid, heat to 45°C and stir for 0.5h, after cooling to room temperature, the solvent is evaporated under reduced pressure to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com