Ornidazole injection and preparation method thereof
An ornidazole injection and a technology for injection, which are applied in directions such as pharmaceutical formulations, drug delivery, and antibacterial drugs, can solve the problems of excessive insoluble particles, strong vascular irritation, potential safety hazards, and the like, and achieve a simple preparation method, suitable for The effect of safety in industrial production and clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
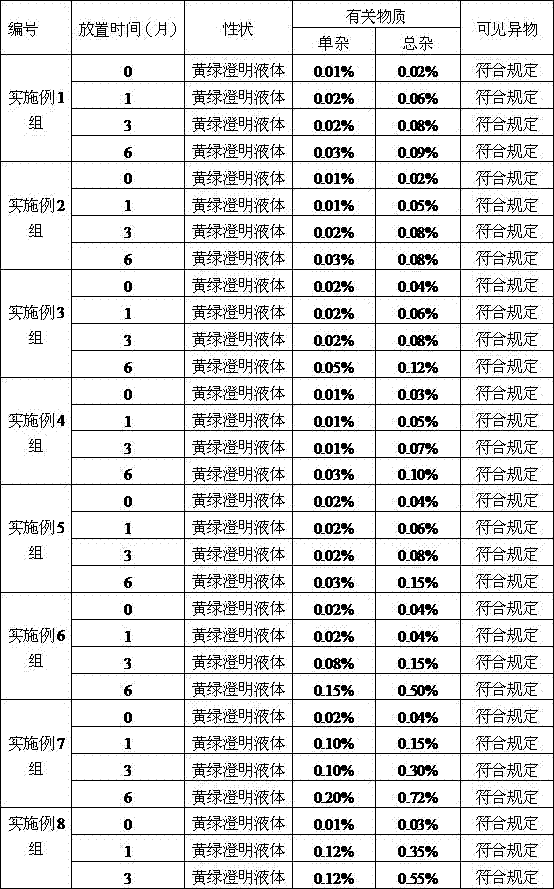
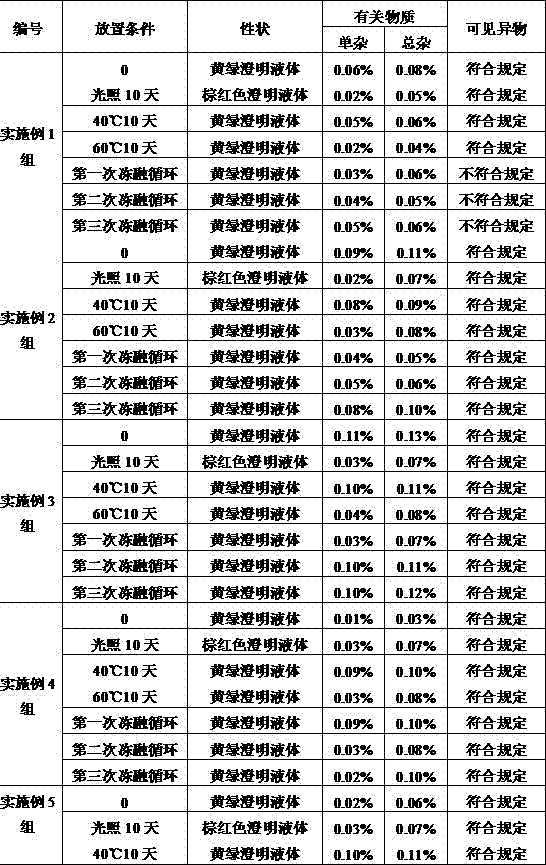
Embodiment 1
[0029] An ornidazole injection, wherein the content of ornidazole is 450g, and the remainder is added with a solvent to make the total volume 3000ml. The solvent is a mixed solution of absolute ethanol and polyethylene glycol-15-hydroxystearate, wherein the mass ratio of polyethylene glycol-15-hydroxystearate is 4%.
[0030] Its preparation method adopts the following steps:
[0031] (1) Equipment treatment: Filter the high-heat compressed air at 180°C through the liquid distribution tank and pipelines for sterilization, depyrogenation, and water removal, and put the equipment into the sterilization cabinet for high-temperature sterilization;
[0032] (2) Solvent preparation: melt polyethylene glycol-15-hydroxystearate in a water bath at 37°C, add the liquid polyethylene glycol-15-hydroxystearate in the formulated amount to the formulated amount of ethanol, and stir Disperse evenly.
[0033] (3) Prepare the liquid medicine: first inject 80% of the solvent into the preparatio...
Embodiment 2
[0042] An ornidazole injection, wherein the content of ornidazole is 600g, and the remainder is added with a solvent to make the total volume 3000ml. The solvent is a mixed solution of absolute ethanol and polyethylene glycol-15-hydroxystearate, wherein the mass ratio of polyethylene glycol-15-hydroxystearate is 6%.
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0045] An ornidazole injection, wherein the content of ornidazole is 750g, and the remainder is added with a solvent to make the total volume 3000ml. The solvent is a mixed solution of absolute ethanol and polyethylene glycol-15-hydroxystearate, wherein the mass ratio of polyethylene glycol-15-hydroxystearate is 8%.
[0046] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com