Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
A technology of dendritic cells and umbilical cord blood is applied in the preparation of DC vaccines and in the field of tumor vaccine treatment products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of DCs derived from umbilical cord blood
[0018] Preparation of autologous cord blood plasma:
[0019] 1. Aseptically collect a portion of umbilical cord blood from a full-term normal delivery fetus after umbilical cord clamping, anticoagulate with citric acid, and centrifuge in a centrifuge tube for 15 minutes.
[0020] 2. Take about 30mL of the supernatant, put it into a centrifuge tube, continue to centrifuge for 15 minutes, and collect the supernatant plasma.
[0021] 3. Place the collected plasma in a 56°C water bath for 30 minutes to inactivate complement.
[0022] 4. Centrifuge to remove the flocculent sediment in the tube, transfer the supernatant to a new centrifuge tube, aliquot and freeze for later use.
[0023] Isolation of cord blood mononuclear cells:
[0024] 1. Mix the umbilical cord blood separated from the upper layer of plasma with normal saline at a ratio of 1:1, and then mix with 6.0% (w / v) hydroxyethyl starch (HESpan) at...
Embodiment 2
[0036] Example 2 Immunophenotypic detection of cultured cells
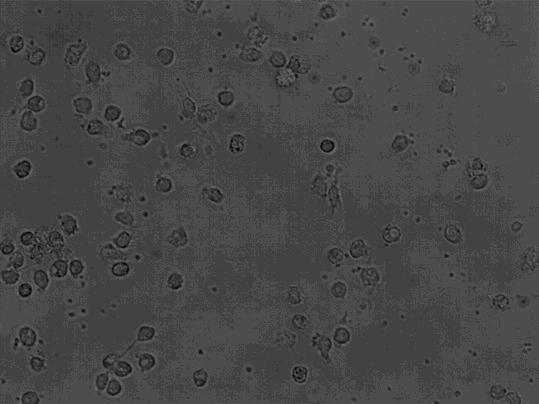
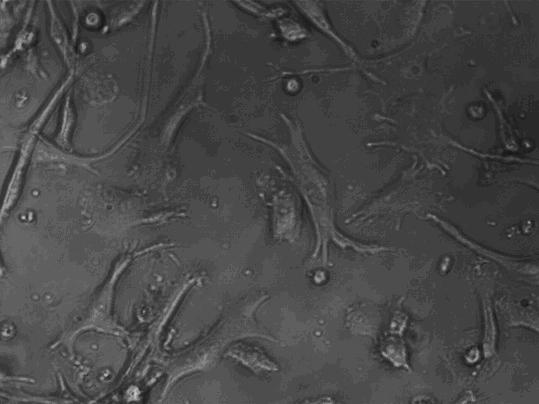
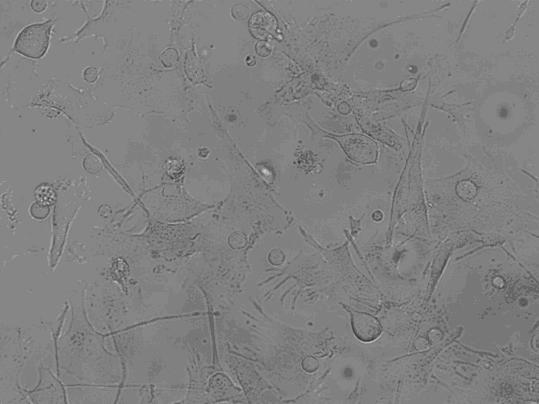
[0037] Take the cells on the 1st, 7th, and 9th day of culture respectively, wash them twice with calcium and magnesium-free PBS, and take 1×10 5 / mL, respectively added to the corresponding FCM tube. Add 5 μl of monoclonal antibodies to be detected, including CD1α, HLA-DR, CD80, CD83, and CD86 antibodies, incubate at 4 °C in the dark for 30 minutes, shake once every 10 minutes, so that the cells can fully contact with the antibodies. Washed twice with PBS, resuspended in 400 μl of PBS, and detected by flow cytometer FASCSCalibur (BD Biosciences), the results are shown in Figure 2, Table 1, image 3 .
[0038]
[0039] Table 1 Immunophenotype of DCs at different culture times
[0040] Training time Day 1 day 7 Day 9 CD1α+ 0.84±0.566% 23.33±2.17% 13.46±1.97% CD83+ 27.37±2.11% 38.57±1.99% 40.88±2.60% HLA-DR+ 76.21±3.09% 82.63±1.62% 89.01±2.59% CD80+ 8.01±2.01% 46....
Embodiment 3
[0042] Example 3 Mixed Lymphocyte Reaction (MLR)
[0043] 1. Take the DCs cultured on day 9, suspend them with AIM-V lymphocyte medium, and adjust the cell concentration to 1×10 6 cells / ml, first treated with mitomycin 25 μg / ml for 45 minutes, and washed with PBS more than 3 times.
[0044] 2. Adjust the DC density to 1×10 5 cells / ml, mixed with T lymphocytes of the corresponding culture time at the ratio of (DC: lymphocytes) 1:10, 1:20, 1:50, 1:100, no DC was added to negative wells, and 3 cells were set in each group Duplicate wells and culture for 72 hours.
[0045] 3. CCK-8 method to detect cell viability: Add human CCK-8, culture for 4 hours, measure OD value with enzyme-linked immunosorbent detector at 450 nm and record the results, and use the average value of 3 wells to count the proliferation rate of T lymphocytes. And calculate its proliferation index SI. SI = OD value of test well / OD value of control well. The test results are shown in Table 2.
[0046]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com