Novel coronavirus(2019-nCoV) ORF1ab gene nucleic acid detection kit
A coronavirus and kit technology, applied in the field of biotechnology and molecular diagnosis, can solve the problems of high cost, long time-consuming 2019 novel coronavirus, failure to form a rapid and complete 2019 novel coronavirus detection system, etc., and achieve high specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106]Example 1 Novel coronavirus ORF1ab gene detection kit
[0107] The kit consists of PCR reaction solution, PCR enzyme system, internal standard, positive control substance, negative control substance and DEPC water. The PCR reaction solution consists of 5×RT-PCR Buffer, primers and probes, and dNTPs; the PCR enzyme system It consists of reverse transcriptase, RNase inhibitor, hot-start DNA polymerase and enzyme diluent; the positive control is prepared from a pseudovirus containing the ORF1ab target sequence, the negative control is normal saline, and the internal standard is containing the internal target Sequenced pseudoviruses were prepared.
[0108] The final concentration of the primer in the amplification system is 800nM, and the final concentration of the probe is 200nM.
[0109] The reaction system of the kit is 25 μL, specifically as follows: 3 μL of PCR enzyme system, 17 μL of PCR reaction solution, 2-5 μL of nucleic acid template, and supplemented with DEPC wa...
Embodiment 2
[0110] Example 2 The operation and result determination of the kit
[0111] (1) Extraction of viral genomic DNA
[0112] Use nucleic acid extraction or purification reagents (product number: DA0623) from Sun Yat-Sen University Daan Gene Co., Ltd. to extract nucleic acids in the sample processing area according to the instructions. Before extraction, each sample was extracted by adding an internal standard at a ratio of 50:1. Both the negative control substance and the positive control substance are involved in the extraction, as the quality control of the environment and PCR detection reagents.
[0113] (2) Preparation of reaction system
[0114] The kit of Example 1 was used to carry out the following experiments. After the PCR reaction solution of the kit was completely dissolved at room temperature, it was shaken and mixed quickly. The 25 μL PCR reaction system was: 17 μL of PCR reaction solution, 3 μL of enzyme system, and 5 μL of template.
[0115] (3) PCR amplificatio...
Embodiment 3
[0128] Example 3 Kit Specific Detection
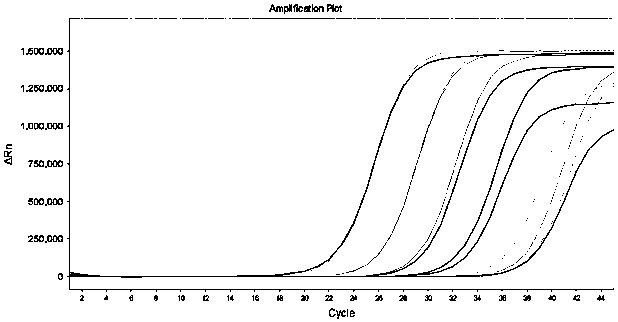
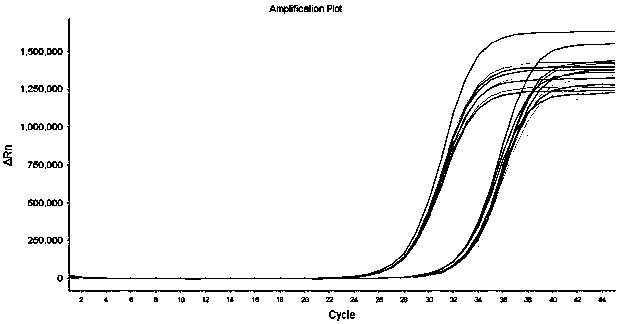
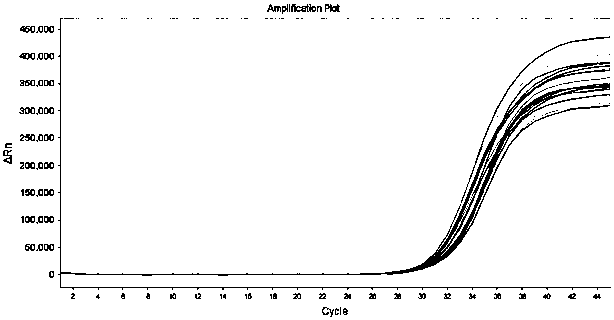
[0129] The kit described in Example 1 is used for influenza A H3N2, H7N9, influenza B Yamagata, respiratory syncytial virus type A, adenovirus type 1, type 3, enterovirus type A, human metapneumovirus, Epstein-Barr virus, Human cytomegalovirus, rotavirus, norovirus, Mycoplasma pneumoniae, Mycobacterium tuberculosis, coronavirus (HKU1, OC43, NL63, 229E), SARS pseudovirus, MERS pseudovirus) and novel coronavirus (2019-nCoV) There was no detection of FAM channel fluorescence during the detection; however, the positive samples for the novel coronavirus were detected as positive. It shows that the specificity of the kit of the present invention is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com