Solid- and solution-phase synthesis of heparin and other glycosaminoglycans
a glycosaminoglycan and solid-phase technology, applied in the field of biopolymers, can solve the problems of unidentified ligands, complex structure of polysaccharides, and inability to understand the relationship between structure and function of hlgags
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Overview of the Present Invention
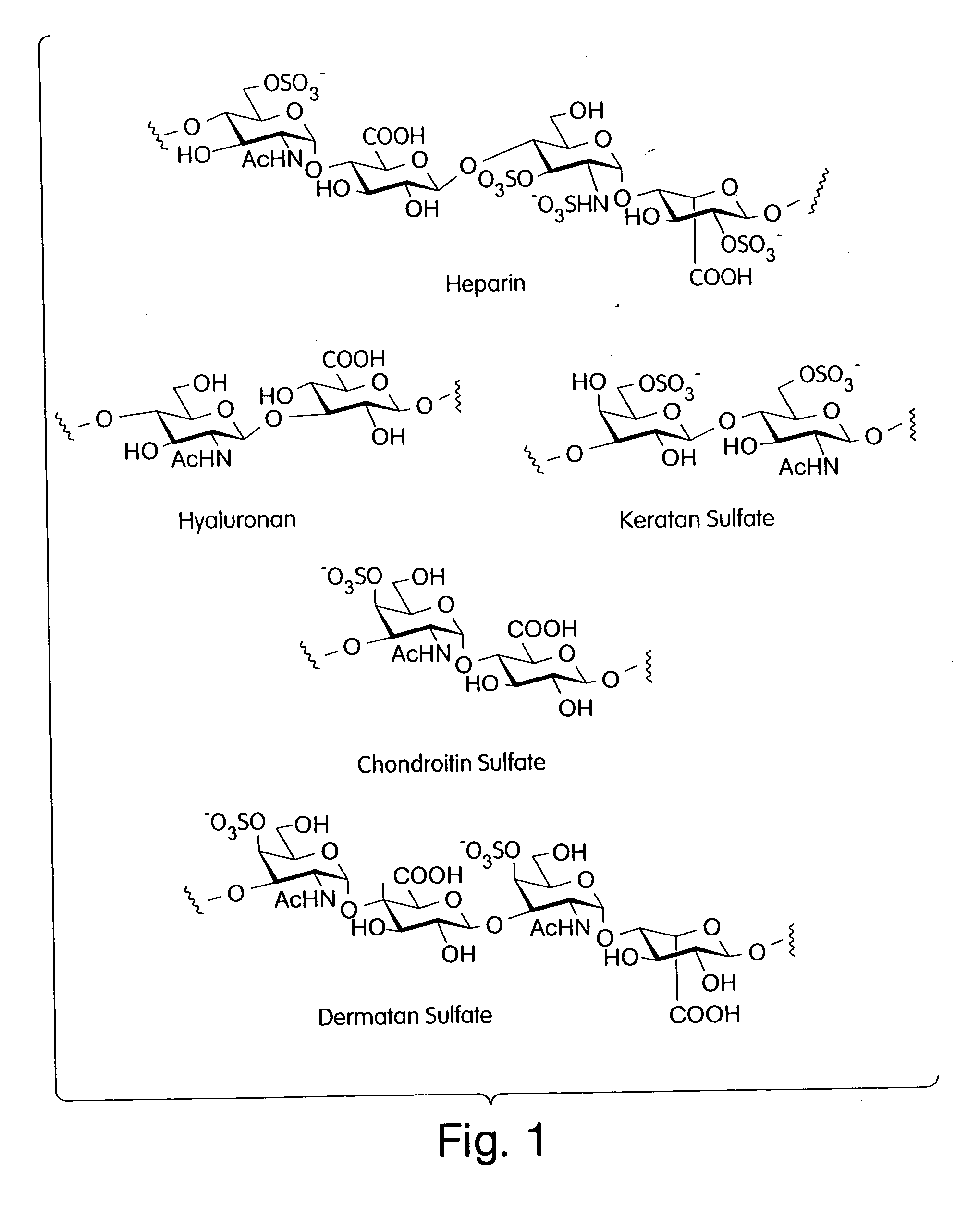
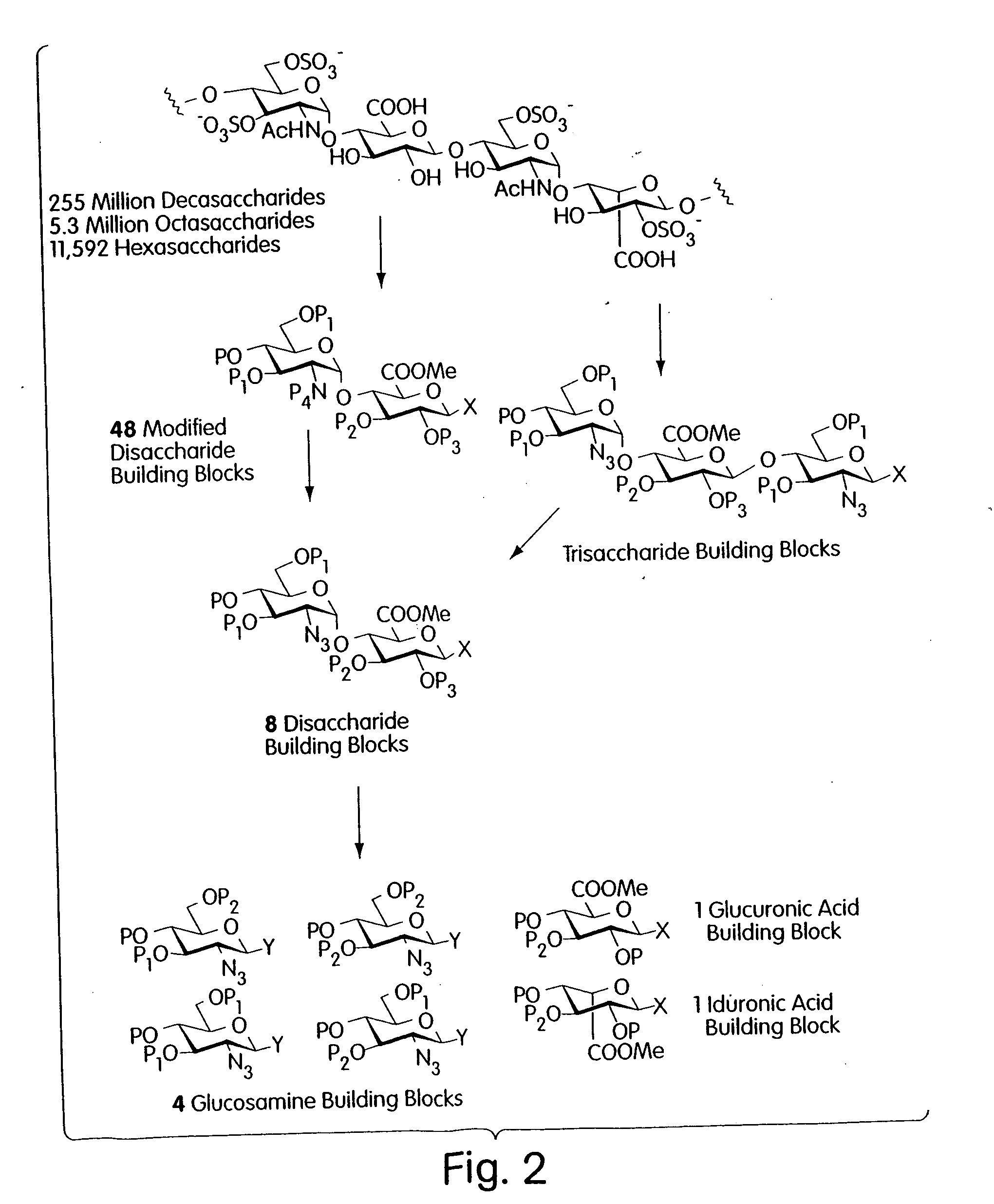
[0035] Described is a modular, general synthetic strategy for the preparation in solution and on a solid support of heparin, heparin-like glycosaminoglycans, glycosaminoglycans and non-natural analogs of each of them. Additionally, the modular strategy provides the basis for the preparation of combinatorial libraries and parallel libraries of defined glycosaminoglycan oligosaccharides. The defined glycosaminoglycan structures may be used in high-throughput screening experiments to identify carbohydrate sequences that regulate a host of recognition and signal-transduction processes. The determination of specific sequences involved in receptor binding holds great promise for the development of molecular tools which will allow modulation of processes underlying viral entry, angiogenesis, kidney diseases and diseases of the central nervous system. Notably, the present invention enables the automated synthesis of glycosaminoglycans in much the same fash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com