ACE2-Fc fusion protein function test method for treating COVID-19
A COVID-19, fusion protein technology, applied in the field of functional testing of ACE2-Fc fusion protein for the treatment of COVID-19
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0030] A method for testing the function of an ACE2-Fc fusion protein for treating COVID-19, specifically comprising the following:
[0031] Step 1. Expression of human wild-type ACE2-Fc fusion protein:
[0032] (1) Cloning the DNA sequence of the extracellular domain (ECD) of human wild-type ACE;
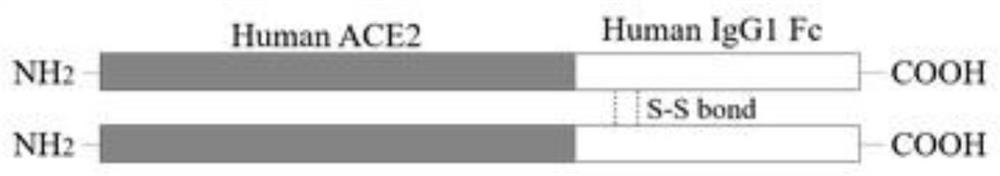
[0033] (2) Fusing the ECD of ACE2 with the Fc part of human IgG1 as follows figure 1 Prolong the half-life of soluble ACE2;
[0034] (3) expressing the ACE2-Fc fusion protein in CHO cells, and using protein A / G agarose to purify the ACE2-Fc fusion protein in the cell culture supernatant;
[0035] Step 2. In vitro testing of the binding function and inhibitory function of the ACE2-Fc fusion protein to the SARS-CoV-2 S1 / RBD protein:
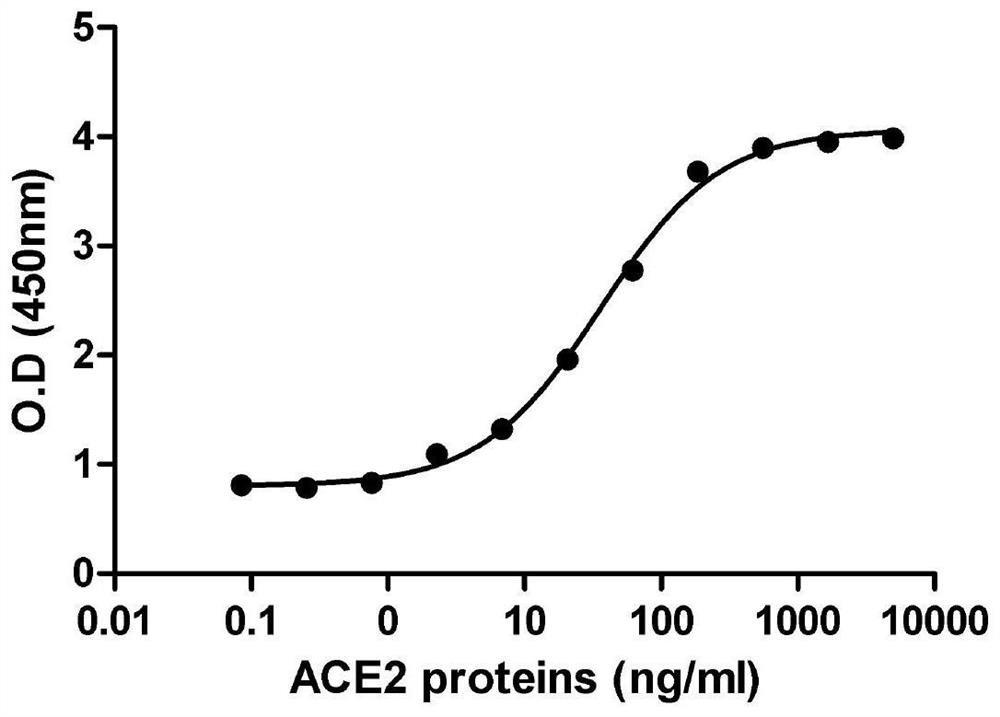
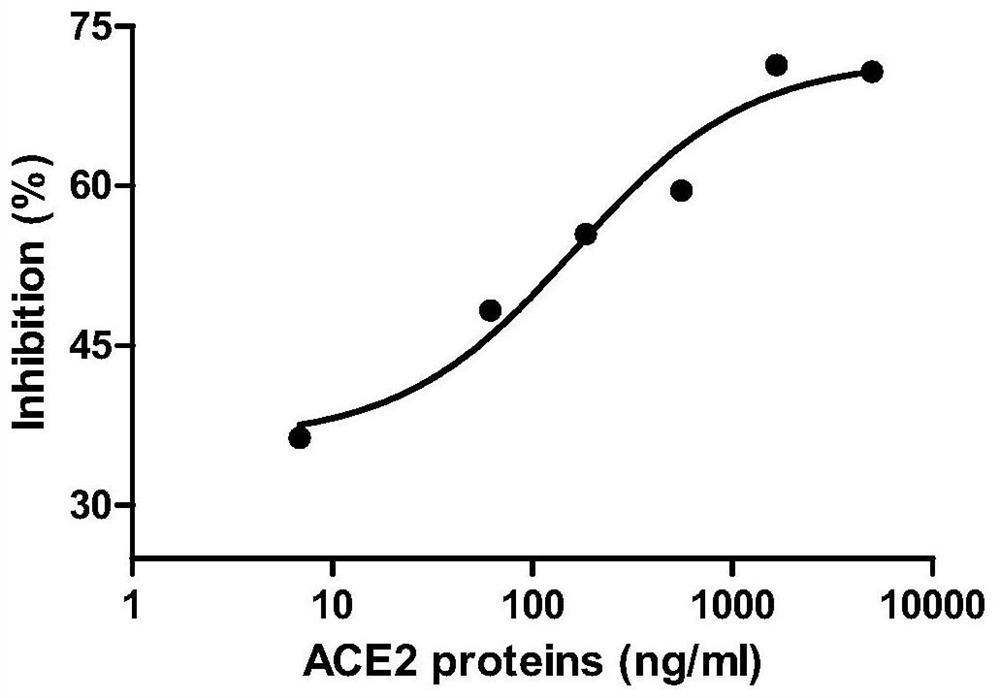
[0036] (4) Coat a 96-well plate with 10ug / ml of SARS-CoV-2RBD-His fusion protein, and then use ACE2-Fc fusion protein to titrate with 2-fold serial dilutions starting from 5ug / ml;
[0037] (5) Record the test data obtained from the titration experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com