Building and application of hydrophobia DNA (deoxyribonucleic acid) vaccine
A DNA vaccine and rabies technology, applied in the field of rabies vaccine, can solve problems such as high cost, pathogenic reversion, and residual virulence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, construction and preparation of eukaryotic expression plasmid pCAGG-RVG
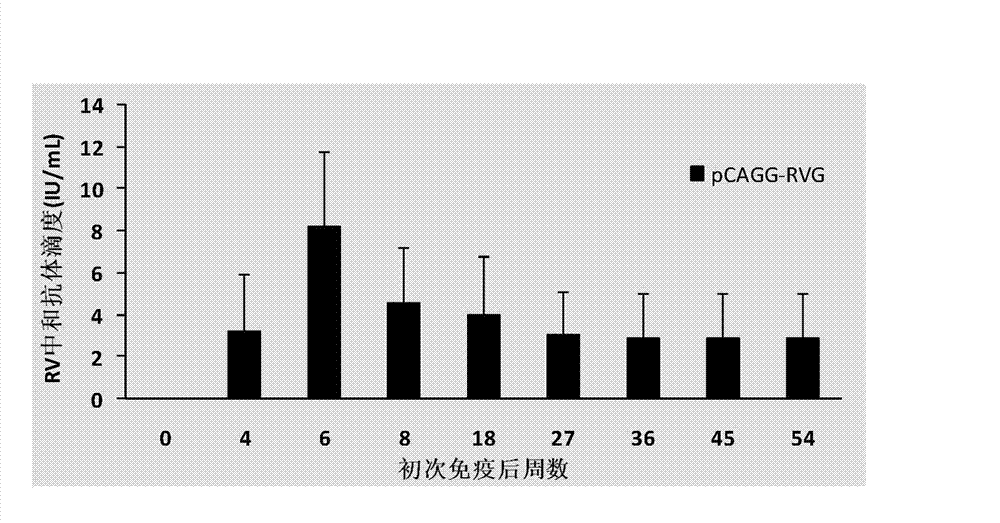
[0022] Optimize the gene sequence of the envelope glycoprotein (G protein) of the attenuated rabies virus vaccine strain ERA (see GenBank number: FJ913470 for its complete genome sequence) according to the mammalian codon bias (see SEG ID No.3 for the original sequence before optimization) . Each amino acid has several codons, which means that these codons can be translated into an amino acid. Mammals have a preference for codons, that is, the translation efficiency of several codons of an amino acid in mammals is high and some are low. In this article, "optimization" means rewriting all the codons of amino acids to the codons in mammals. Codons with high translation efficiency in vivo. The difference between the original sequence and the optimized sequence is that the translated amino acid sequence remains unchanged, but the nucleic acid sequence changes greatly. See SEG ID No.1 ...
Embodiment 2
[0024] Embodiment 2, transient transfection and indirect immunofluorescence (IFA) detection of eukaryotic expression plasmid pCAGG-RVG
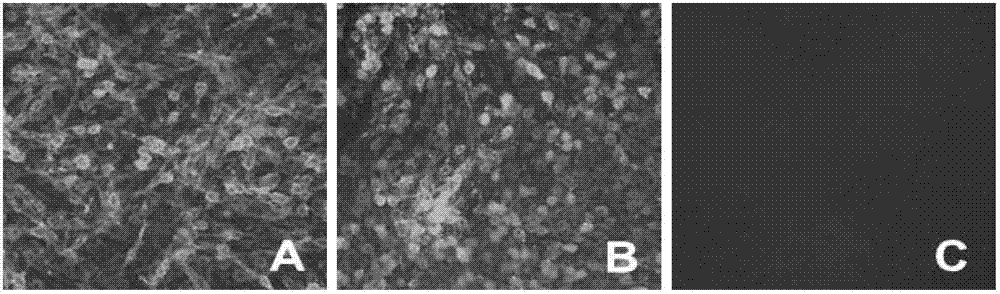
[0025] In order to confirm the antigenicity of pCAGG-RVG expressing the RVG protein, BHK cells were transfected with pCAGG-RVG. Before transfection, BHK cells grown overnight in a 24-well plate with a density of about 80% were prepared, and liposome transfection method (specific operation See Lipofectamine for details TM 2000 instructions) transfected with 0.5 μg of pCAGG-RVG transfected into the above cells, transfected and then 5% CO 2Incubate at 37°C. At the same time, BHK cells were infected with attenuated RV vaccine strain ERA at an MOI of 0.1 as a positive control; BHK cells were transfected with 0.5 μg of pCAGGS as a negative control. 36 hours after transfection, wash the cells with PBS 3 times, fix the cells with pre-cooled 3% paraformaldehyde for 30 min; wash 3 times with PBS, block with 1% BSA for 20 min; wash with PBST (0.05% Tw...
Embodiment 3
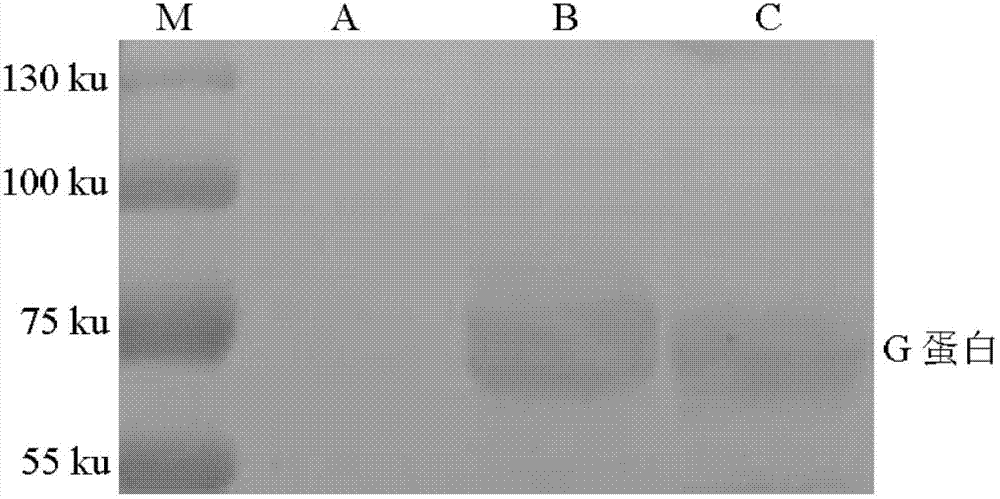
[0027] Embodiment 3, western blot (Western blot) analysis
[0028] In order to analyze whether the recombinant eukaryotic expression plasmid could express RVG protein, 2 μg of pCAGG-RVG was used to transfect monolayer BHK cells spread in 6-well plate. 36 hours after transfection, harvest and lyse the cells, add an equal volume of 2×SDS lysis buffer, boil for 10 minutes, centrifuge at 10,000×g for 10 minutes, take the supernatant for SDS-PAGE (Bio-Rad) electrophoresis; Print (Bio-Rad) onto a nylon membrane (Ameresco), block overnight with 5% skim milk, wash with PBST and add 1:100 times diluted mouse anti-RV serum as the primary antibody, act at room temperature for 1 h, wash with PBST, add 1: Horseradish peroxidase (HR)-labeled rabbit anti-mouse IgG secondary antibody (Sigma NO.A9044) was diluted 2500 times in PBST for 1 hour at room temperature, washed with PBST, and then developed with DAB.
[0029] Western blotting showed a specific 67ku detection protein, consistent with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com