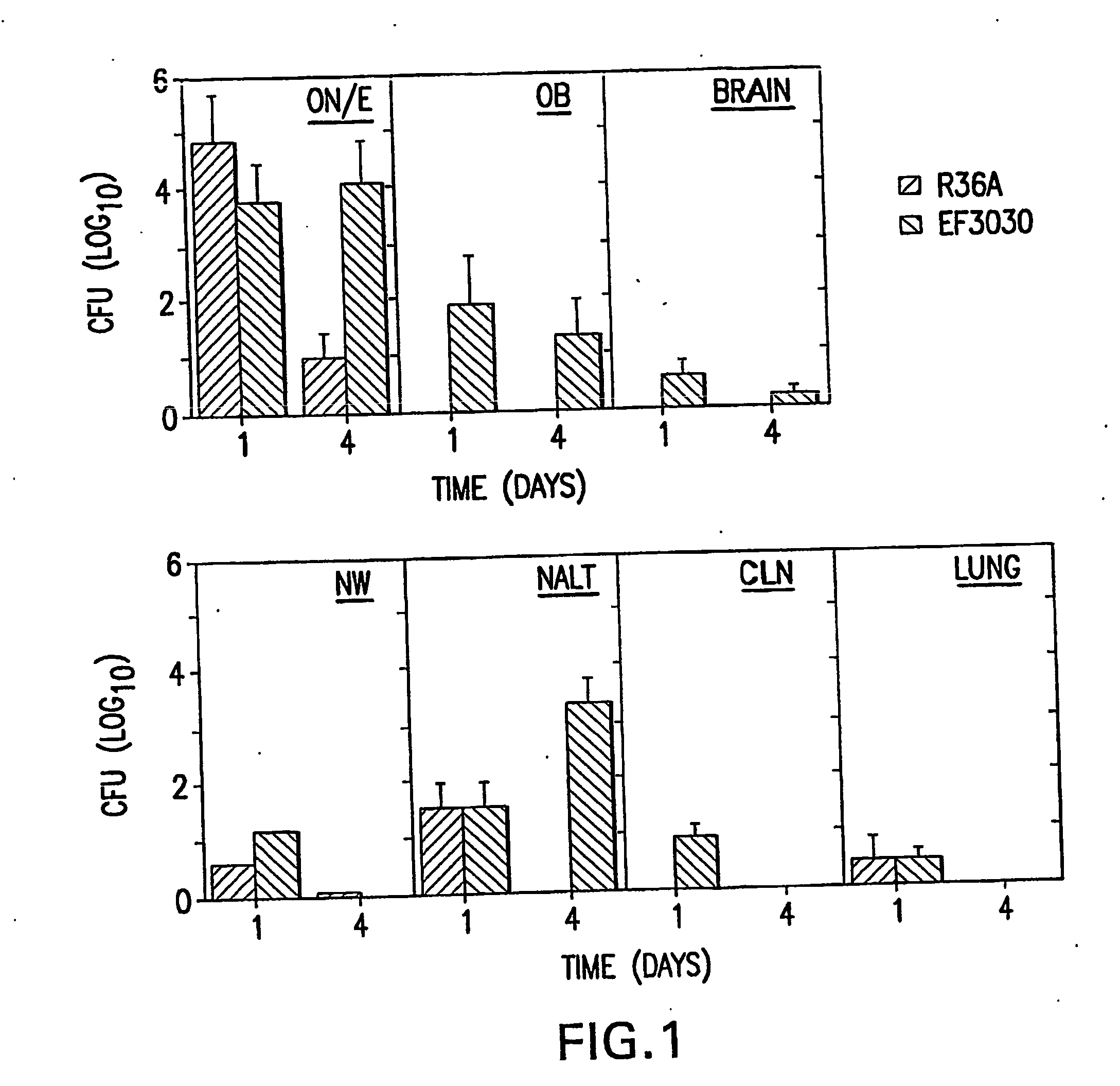
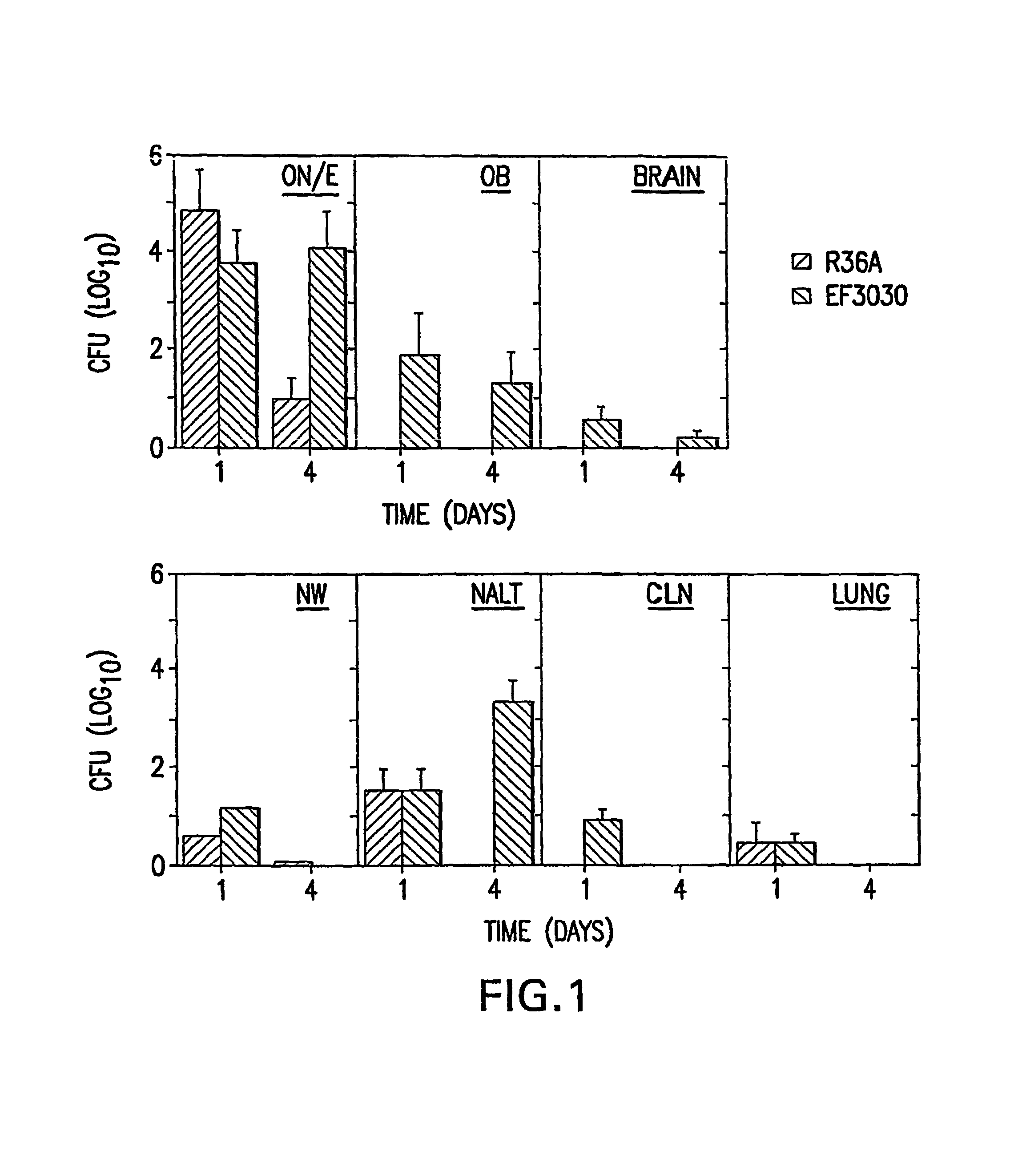
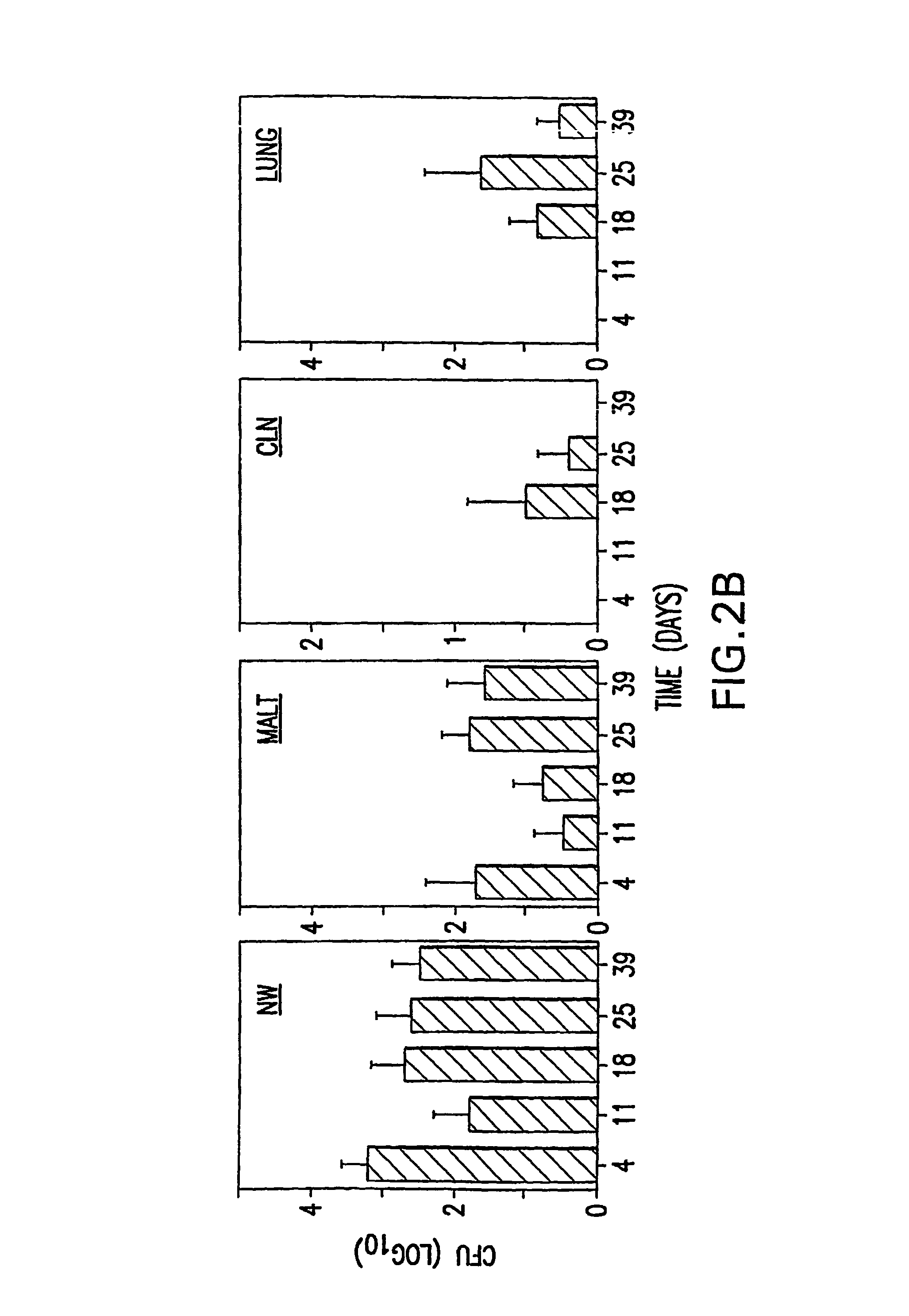
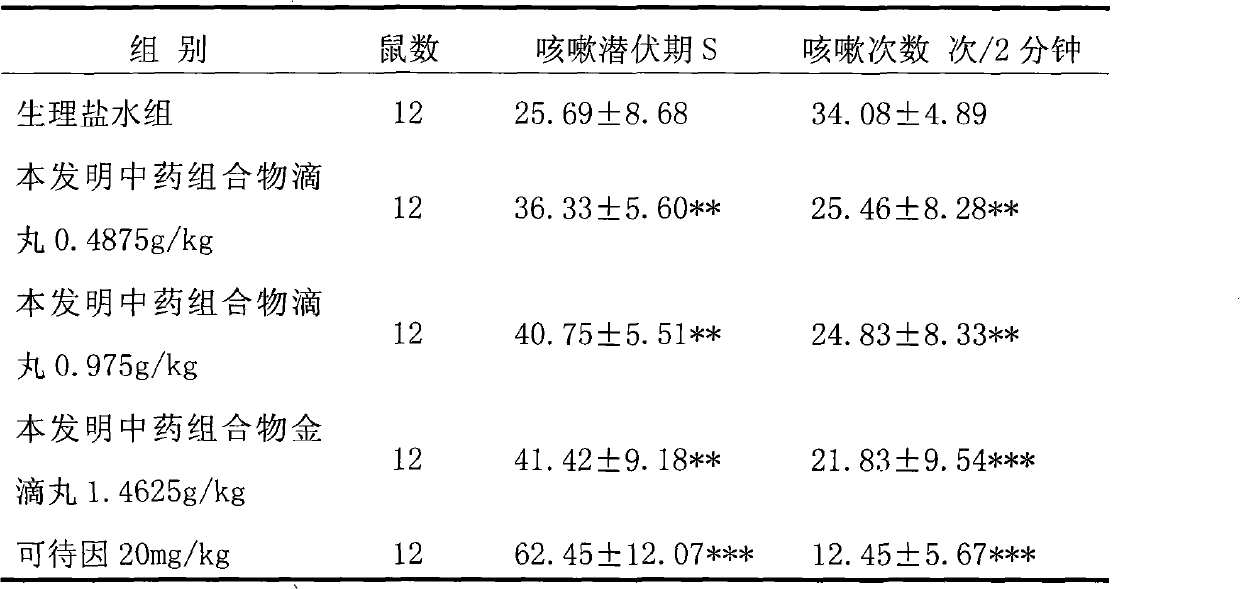
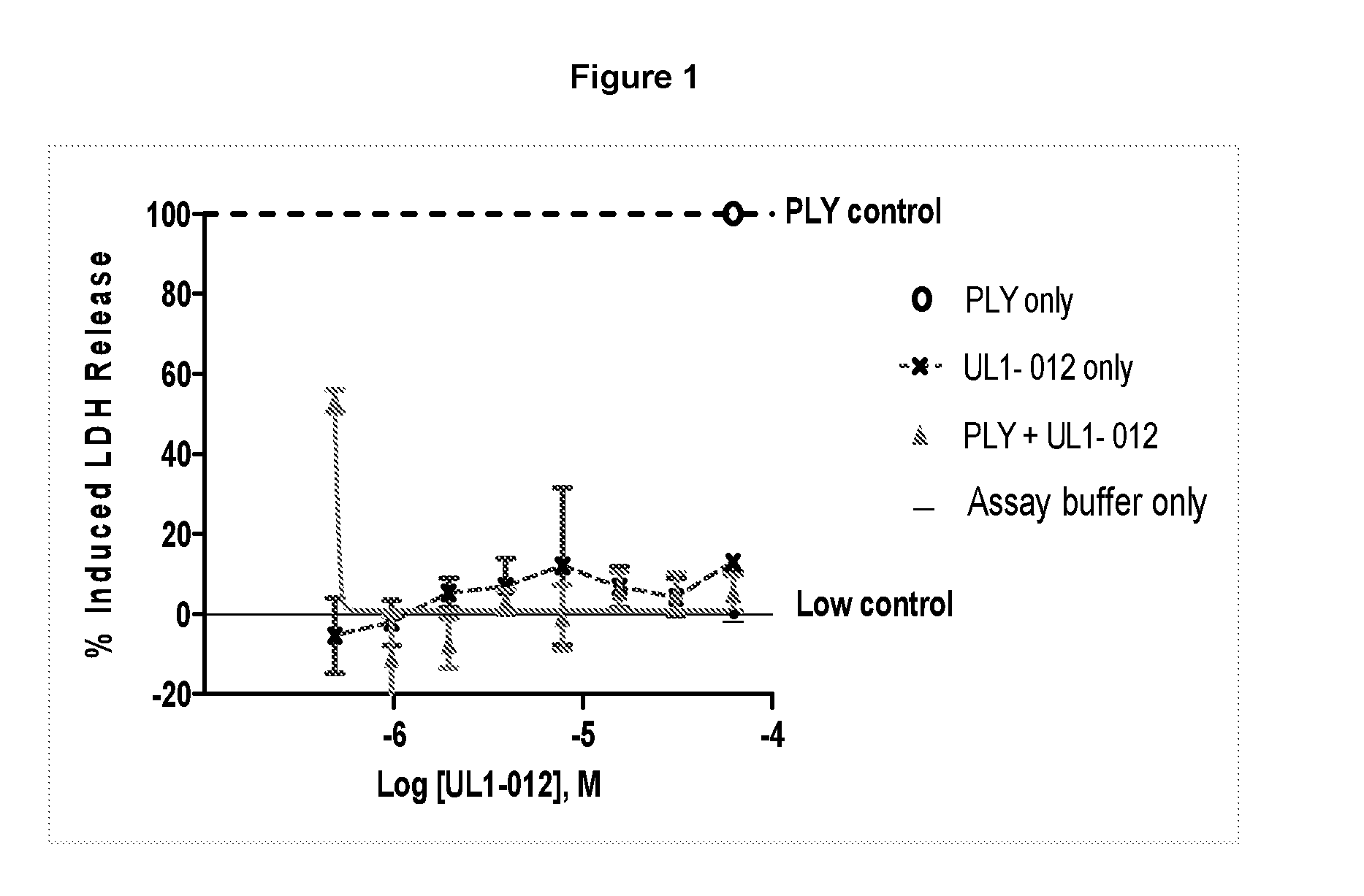
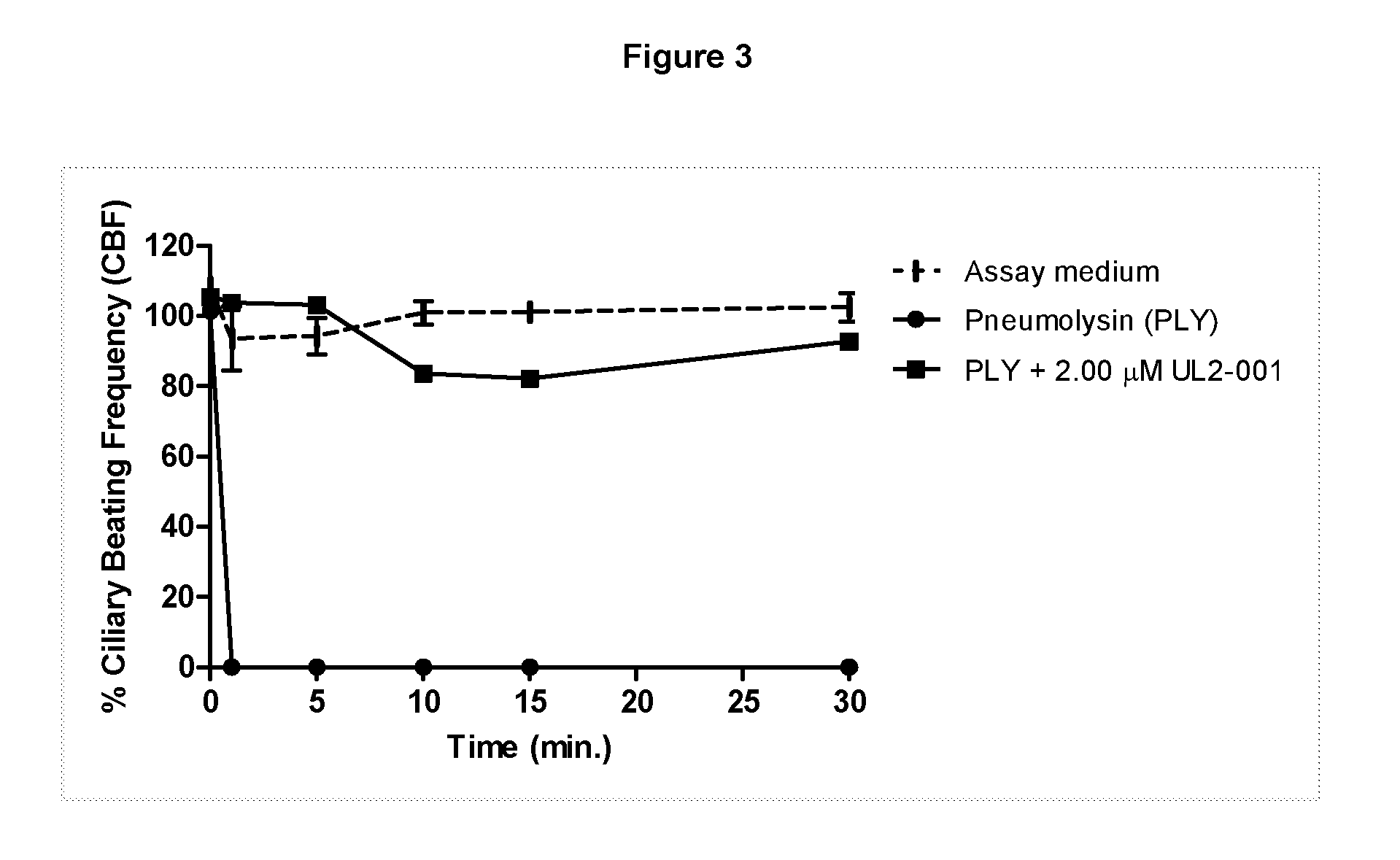
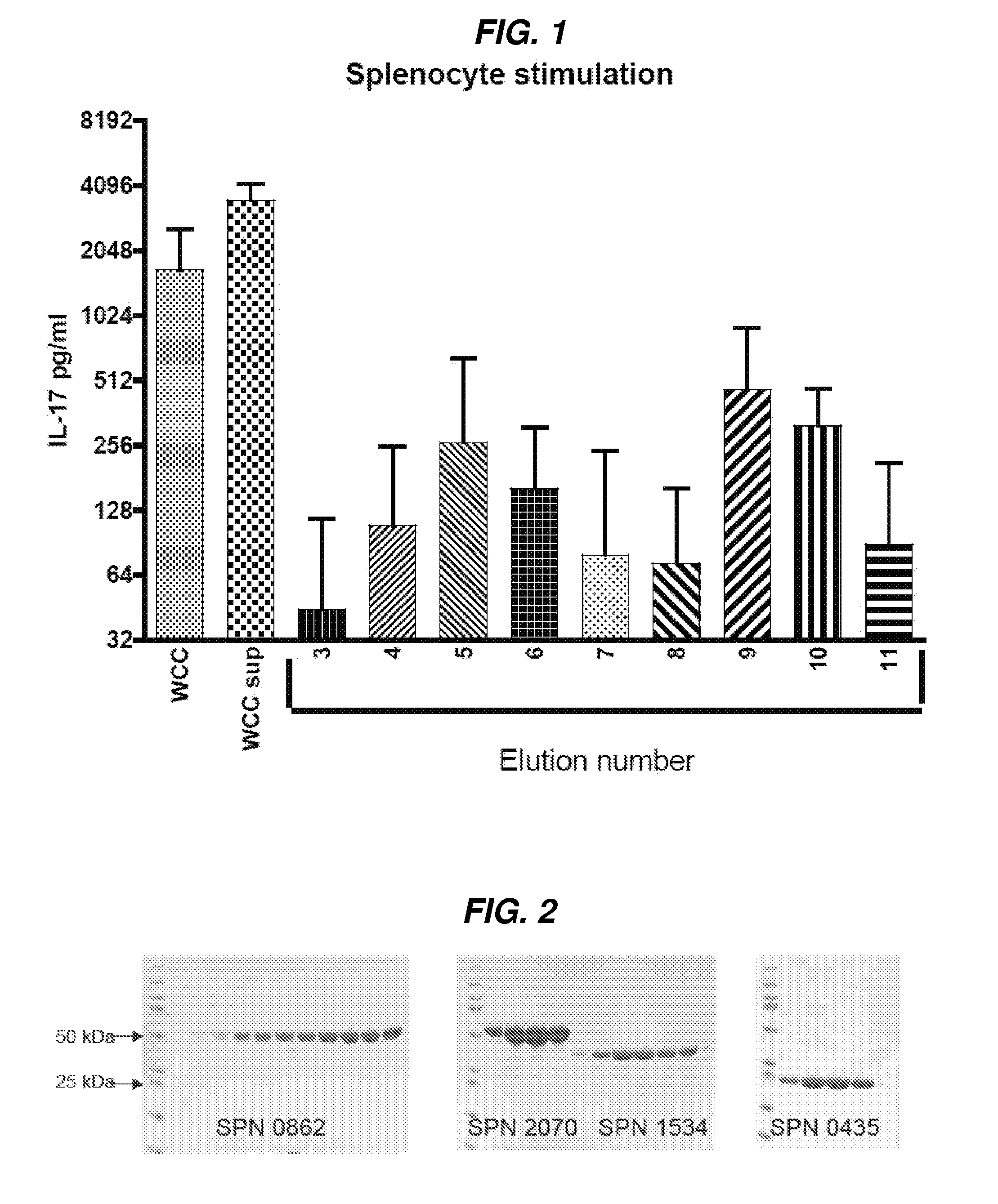
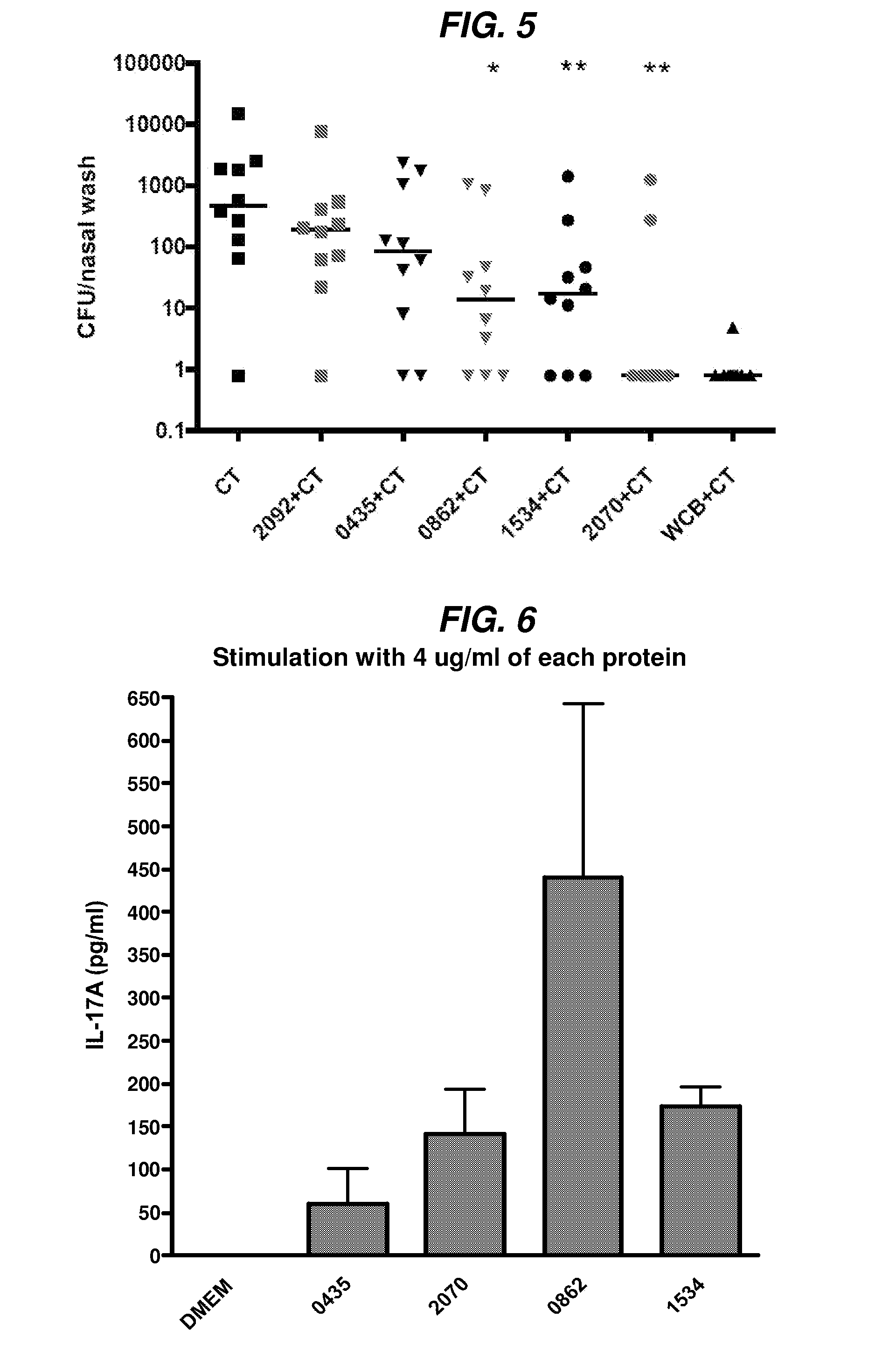
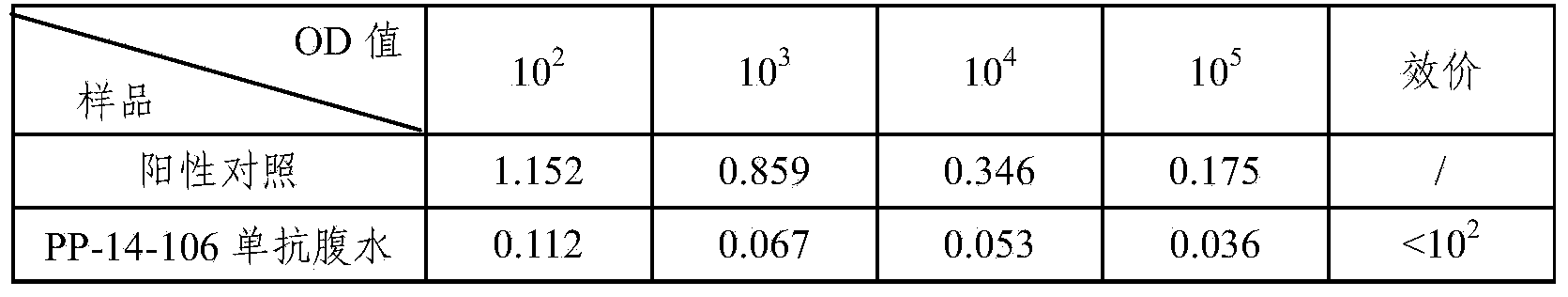Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Pneumococcal infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pneumococcal infections are caused by the Streptococcus pneumoniae bacteria, and range from mild to severe.
Pneumococcal vaccine and uses thereof
InactiveUS20120052088A1Low toxicitySafe and effective T-cell dependent carrierAntibacterial agentsNervous disorderCoccidiaSurgery
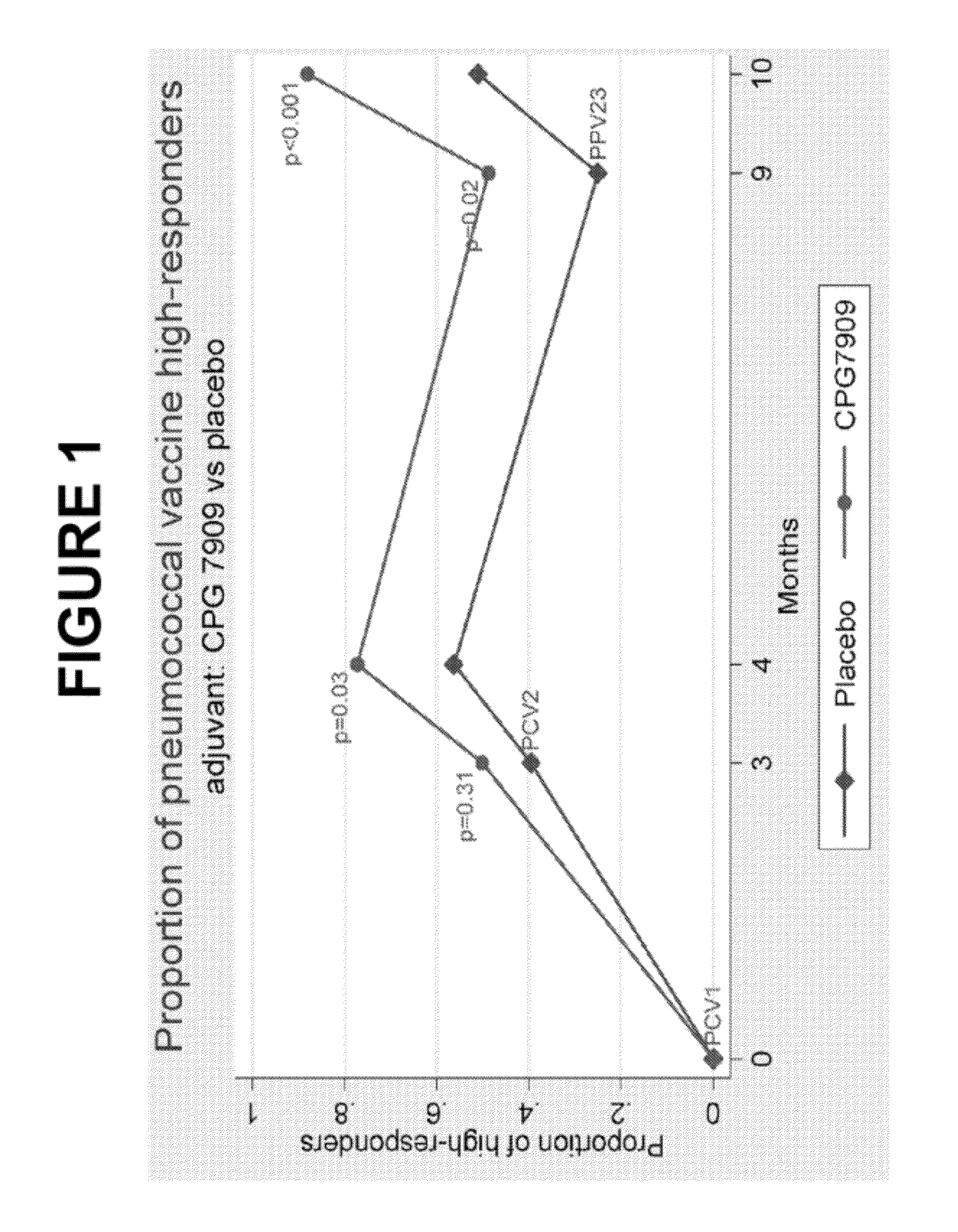
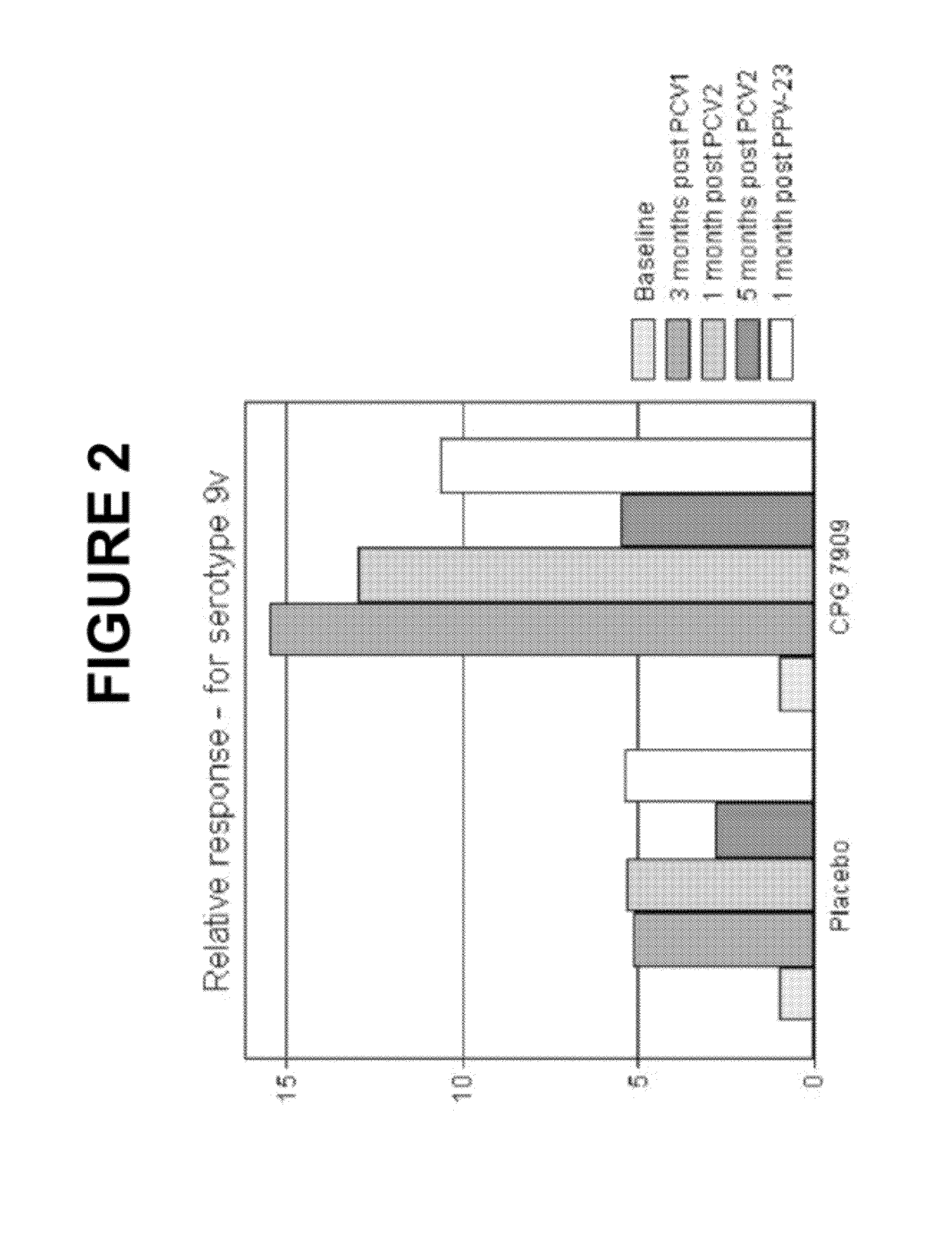
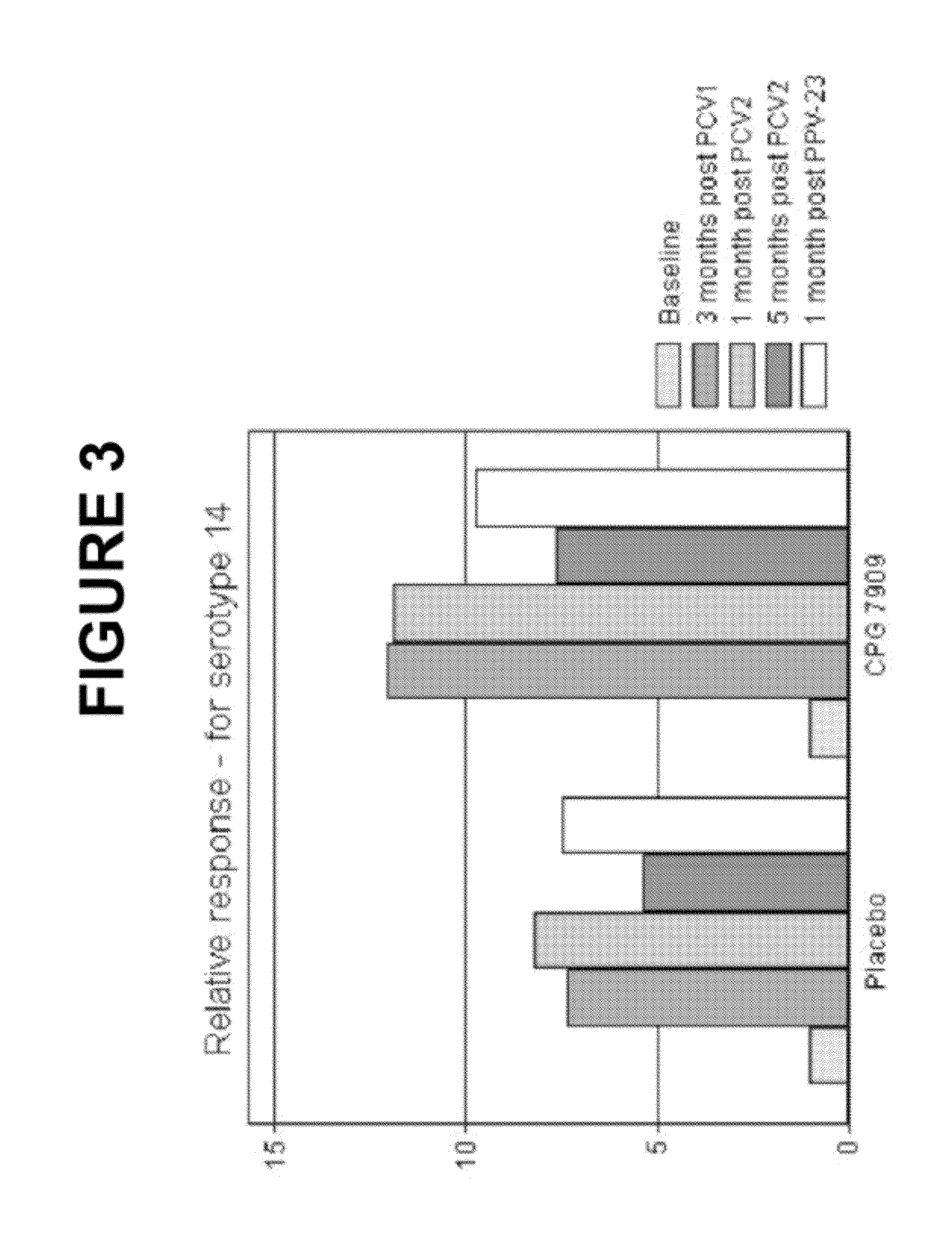
The present invention relates to new pneumococcal vaccines. The invention also relates to vaccination of subjects, in particular immunocompromised subjects, against pneumococcal infections using said novel pneumococcal vaccines.
Owner:COLEY PHARM GRP INC
Immunogenic compositions comprising conjugated capsular saccharide antigens and uses thereof
ActiveUS9492559B2Antibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniae conjugatedPrevnar 13
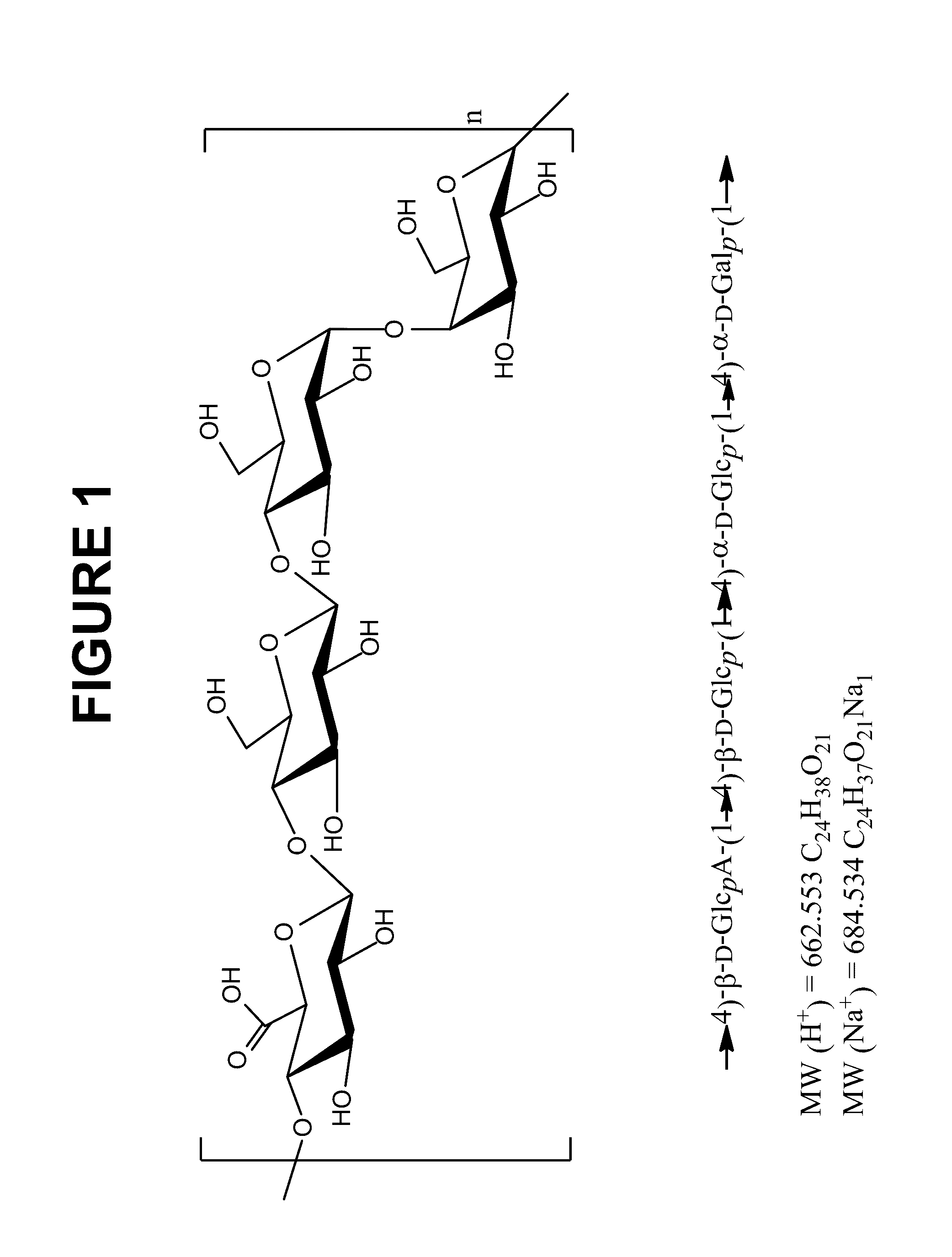
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
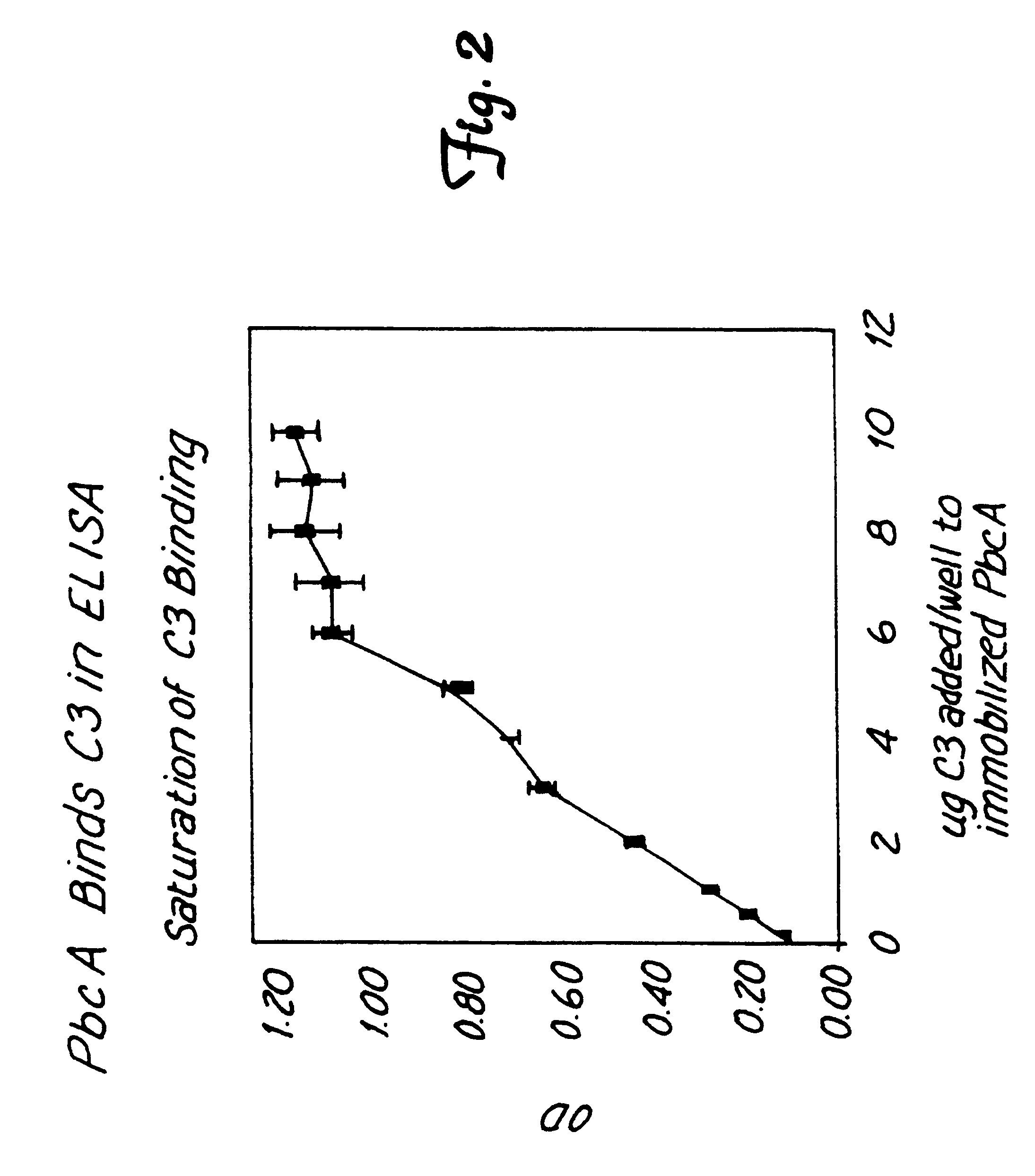
Method for isolating a C3 binding protein of streptococcus pneumoniae
This invention relates to the identification of a human complement C3 binding protein from Streptococcus pneumoniae and to its sequence and to methods for its purification and use. The protein binds but does not degrade or cleave C3 and is implicated in S. pneumoniae virulence. The protein is recognized by antibodies produced by humans recovering from pneumococcal infection.
Owner:RGT UNIV OF MINNESOTA
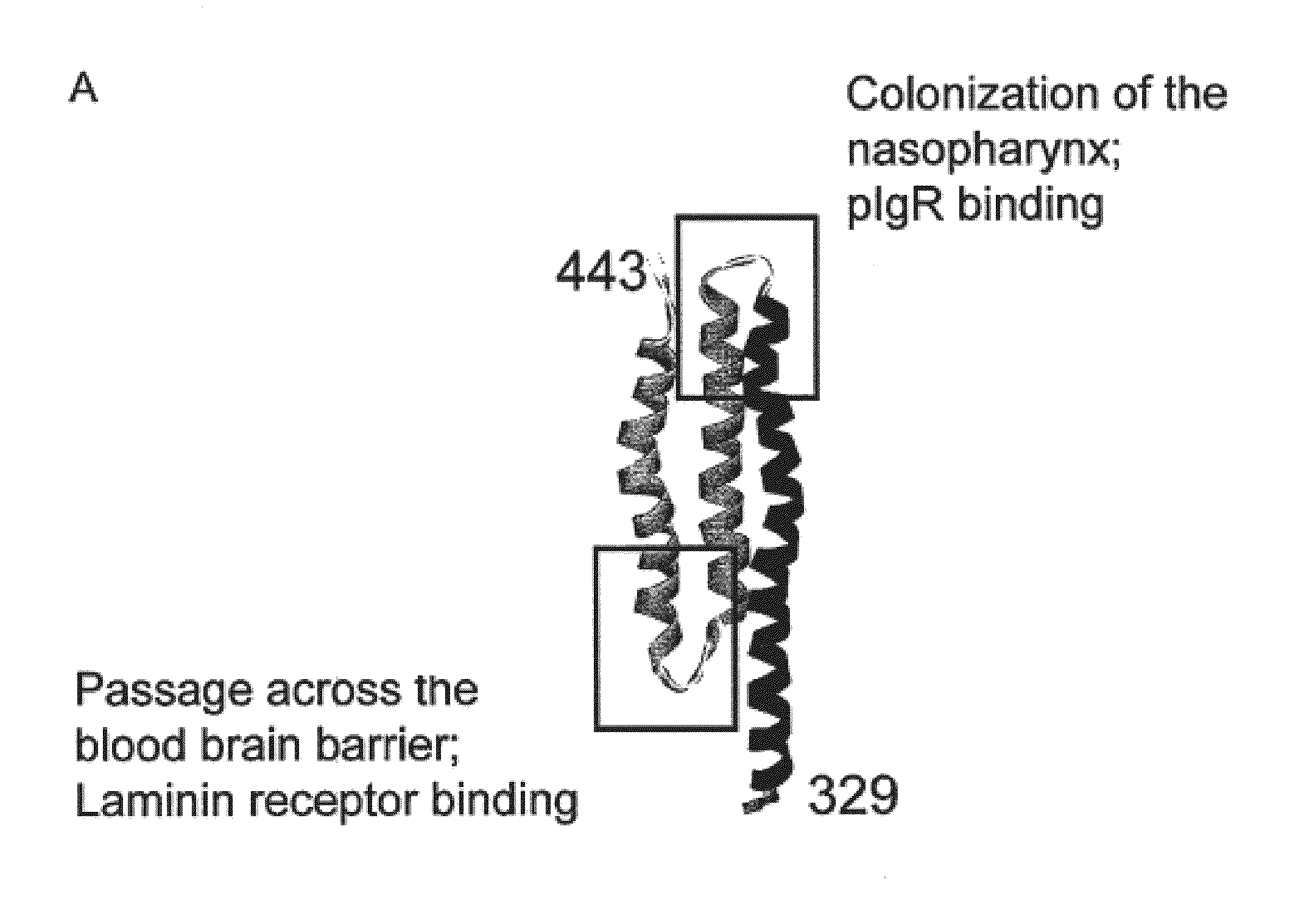
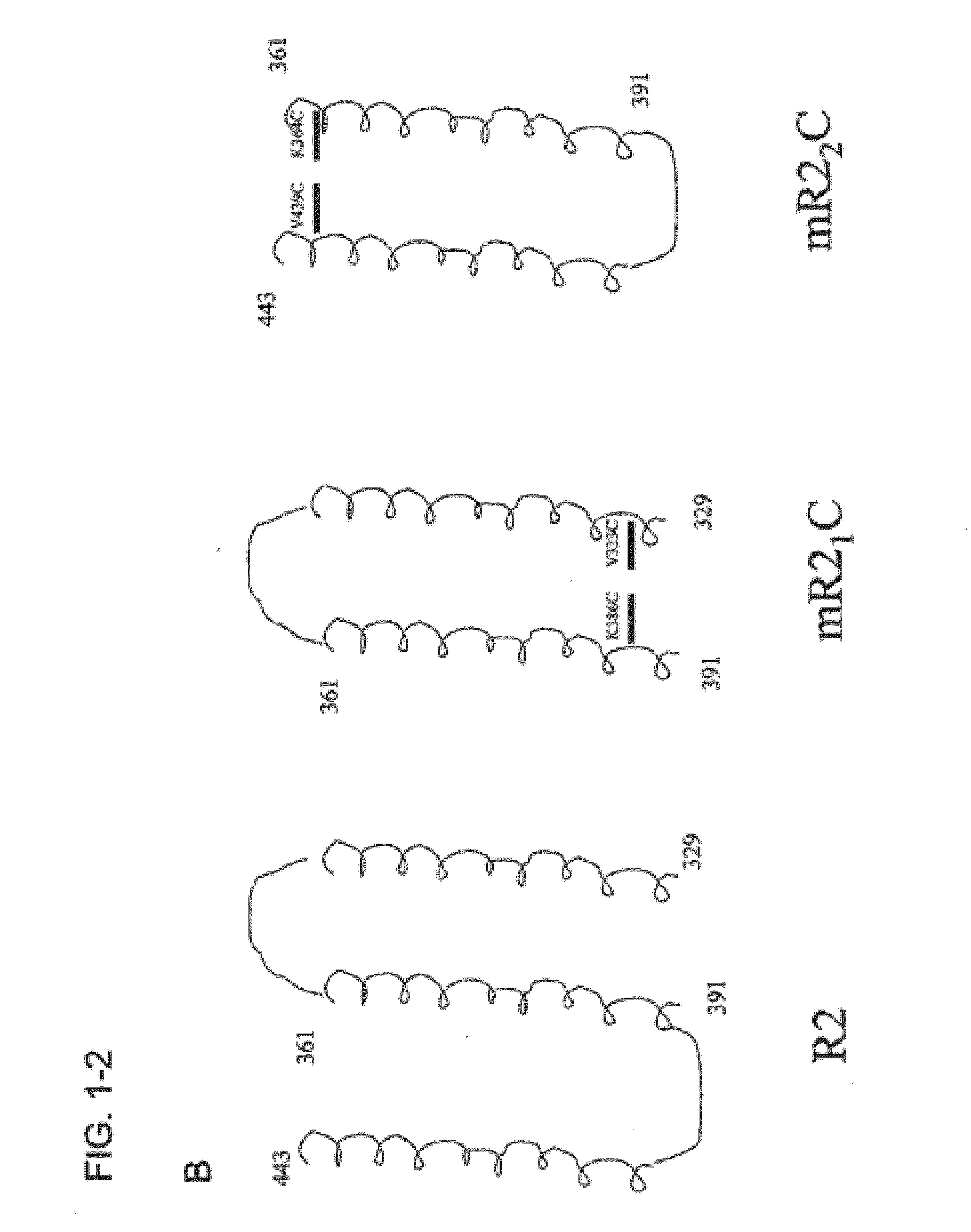
Synthetic streptococcus pneumoniae vaccine
Compositions and methods for preventing and treating pneumococcal infections are provided. Compositions include novel polypeptides comprising an amino acid sequence corresponding to the R2i or R22 domain of CbpA or a consensus sequence of one of these domains, and variants and fragments thereof, wherein the polypeptide is stabilized in a desired conformation, particularly a loop conformation. The polypeptides of the invention may be engineered to comprise a first and a second cysteine residue, thereby resulting in the formation of a disulfide bond that stabilizes the polypeptide in the desired conformation. Alternatively, a polypeptide of the invention may be modified to create a synthetic linkage between a first and second amino acid residue present within the polypeptide, wherein the synthetic linkage stabilizes the polypeptide in the desired conformation. The polypeptides of the invention may further comprise an amino acid sequence for a T cell epitope. Compositions further include isolated nucleic acid molecules that encode the polypeptides of the invention, immunogenic compositions and vaccines comprising the disclosed polypeptides, and antibodies specific for these polypeptides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
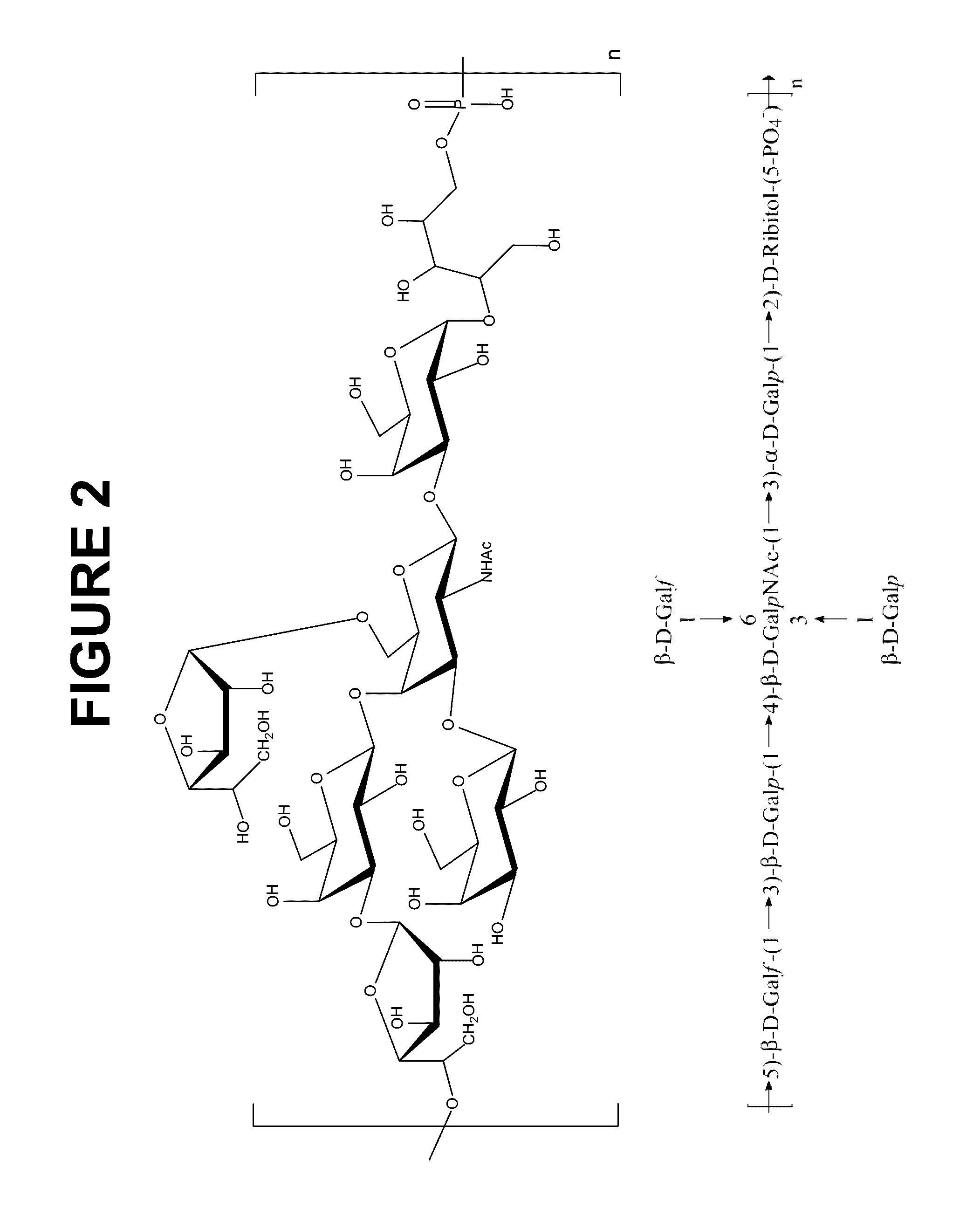
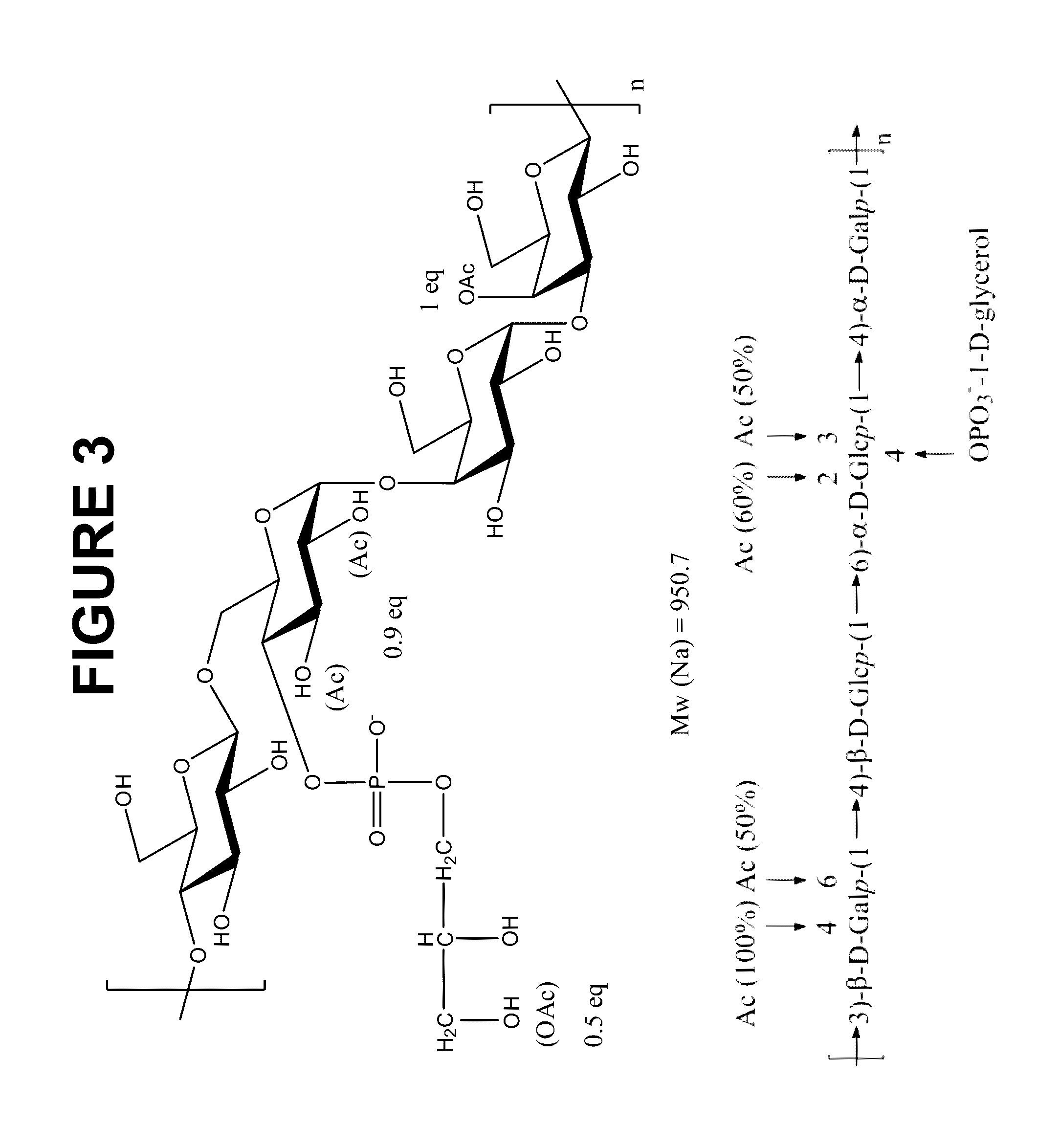
Pneumococcus polysaccharide protein coupling vaccine and its preparing method
The present invention is pneumococus polysaccharide protein coupling vaccine comprising covalently connected pneumococus capsule polysaccharide and recombinant pneumolysin without hemolytic activity modification and its preparation process. The vaccine has pneumolysin without hemolytic activity as protein carrier, no need of eliminating hemolysis toxicity of pneumolysin with formalin and ensured safety, and may be used for infant below 2 yeas old to prevent tympanitis. Owing to the pneumolysin as the self protein, the vaccine has no probable immune interference reaction and strengthened immune protecting effect. The vaccine has cross immunizing protection effect on various kinds of serum type pneumococus and raised immune memory response to pneumococus infection.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Immunogenic Compositions for Use in Pneumococcal Vaccines
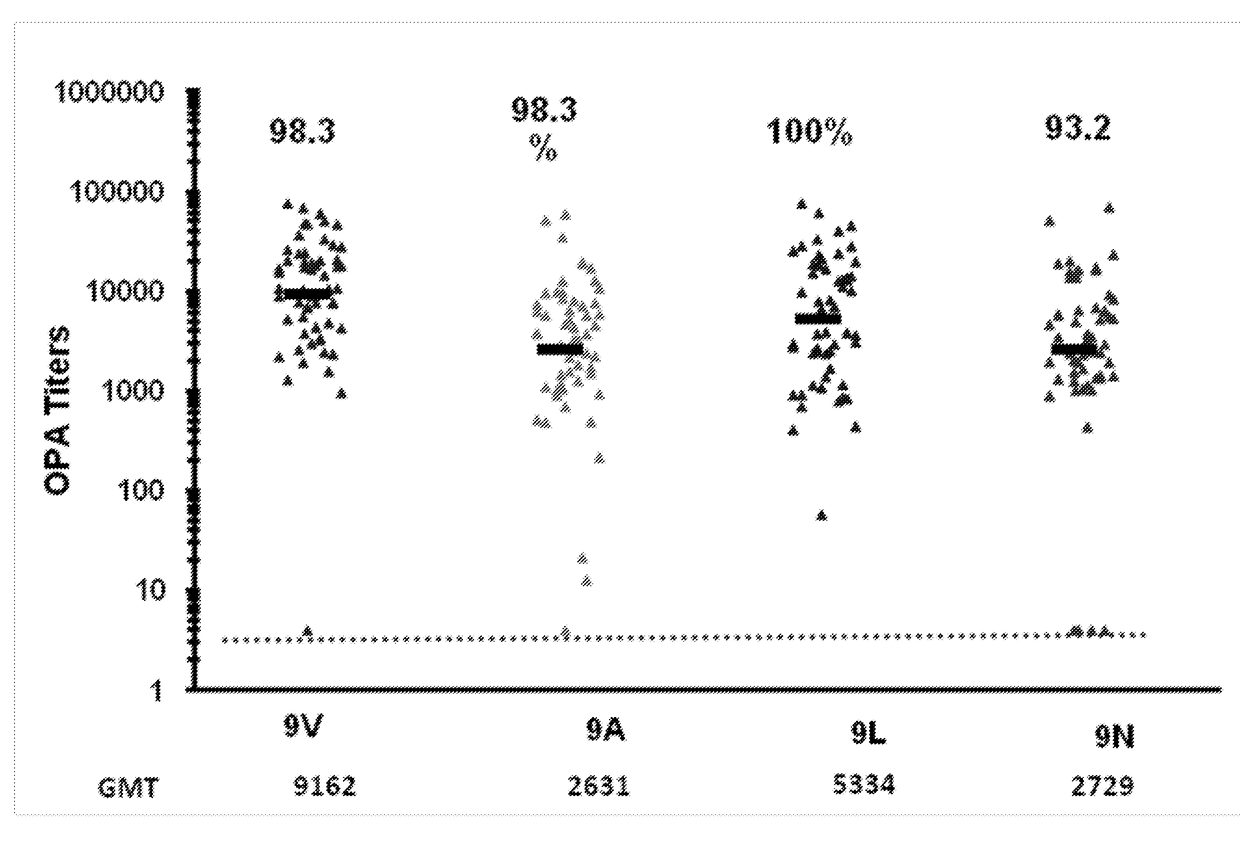
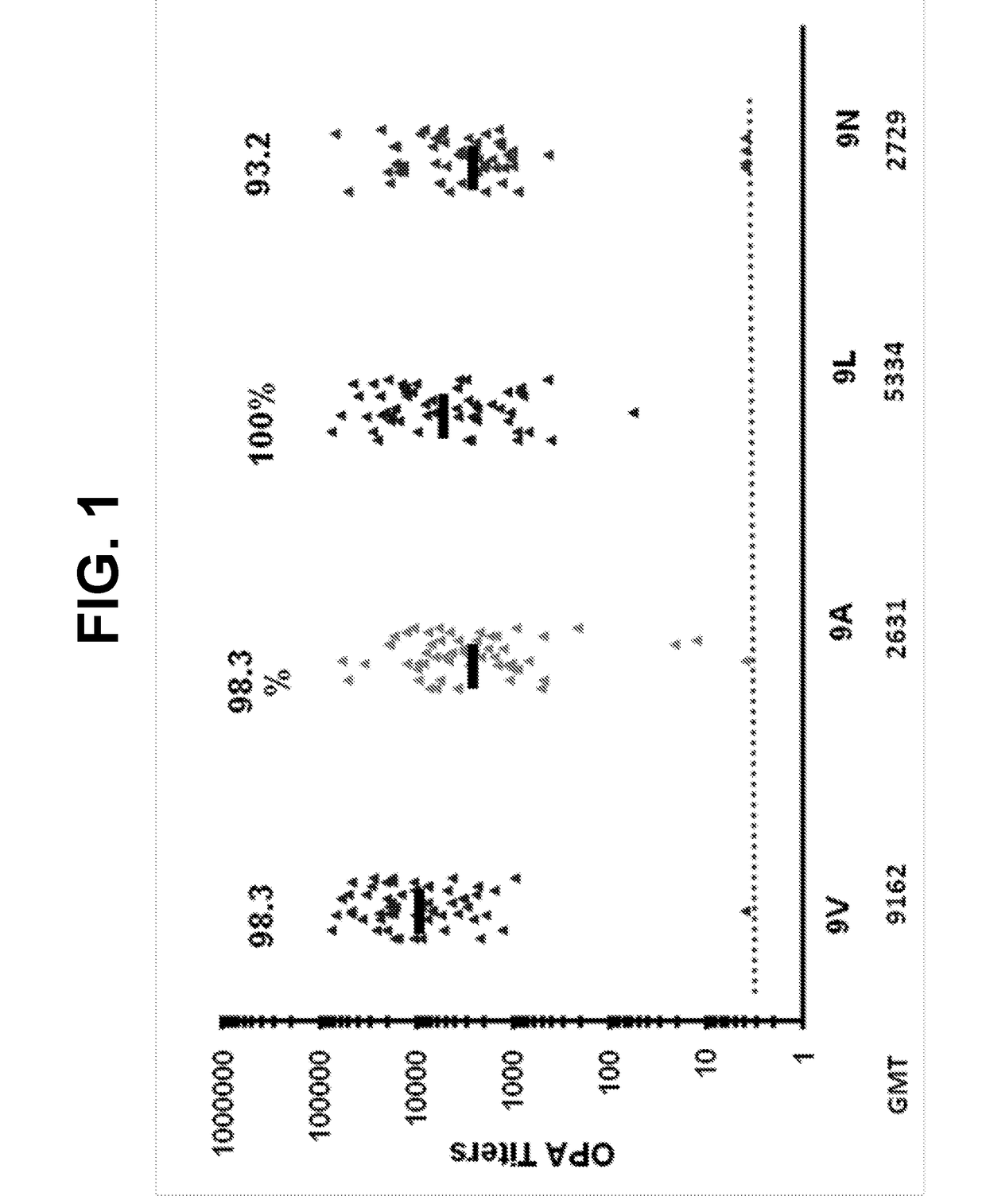
ActiveUS20180000922A1Raise the ratioIncrease OPA titerAntibacterial agentsBacterial antigen ingredientsPneumococcal vaccineImmunogenicity
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 9, while limiting the number of conjugates. The present invention thereforerelates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Compositions and methods for administering pneumococcal DNA
Plasmid DNA encoding at least one pneumococcal antigen or epitope of interest and methods for making and using such a plasmid are disclosed and claimed. The epitope of interest can be PspA or a fragment thereof. Compositions containing the plasmid DNA are useful for administration to a host susceptible to pneumococcal infection for an in vivo response, such as a protective response, or for generating useful antibodies. The inventive plasmid can also be transfected into cells for generating antigens or epitopes of interest in vitro. And the inventive plasmid can be prepared by isolating DNA (coding for: promoter, leader sequence, epitope of interest and terminator), and performing a three-way ligation. More particularly, administration of DNA encoding pneumococcal antigens or epitopes of interest and compositions therefor for eliciting and immunological response against S. pneumoniae, such as a protective response preventive of pneumococcal infection, are disclosed and claimed. Thus, pneumococcal vaccines or immunological compositions, and methods of making and using them, are disclosed and claimed.
Owner:BRILES DAVID E +2
Modulating production of pneumococcal capsular polysaccharide
The invention relates to a method of assessing whether a test compound is useful for alleviating a pneumococcal infection in an animal. This method comprises comparing (a) the degree of phosphorylation of CpsD in pneumococcal cells maintained in the presence of the test compound and (b) the degree of phosphorylation of CpsD in the same type of cells maintained in the absence of the test compound.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Compositions For Reducing Bacterial Carriage And Cnc Invasion And Methods Of Using Same
ActiveUS20070286866A1Reduce and prevent bacterial infectionAntibacterial agentsSenses disorderAntigenNasal cavity
Owner:UAB RES FOUND
Immunogenic Compositions for Use in Pneumococcal Vaccines
ActiveUS20190343946A1Antibacterial agentsBacterial antigen ingredientsStreptococcus mitisImmunogenicity
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 18, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Compositions for reducing bacterial carriage and CNS invasion and methods of using same
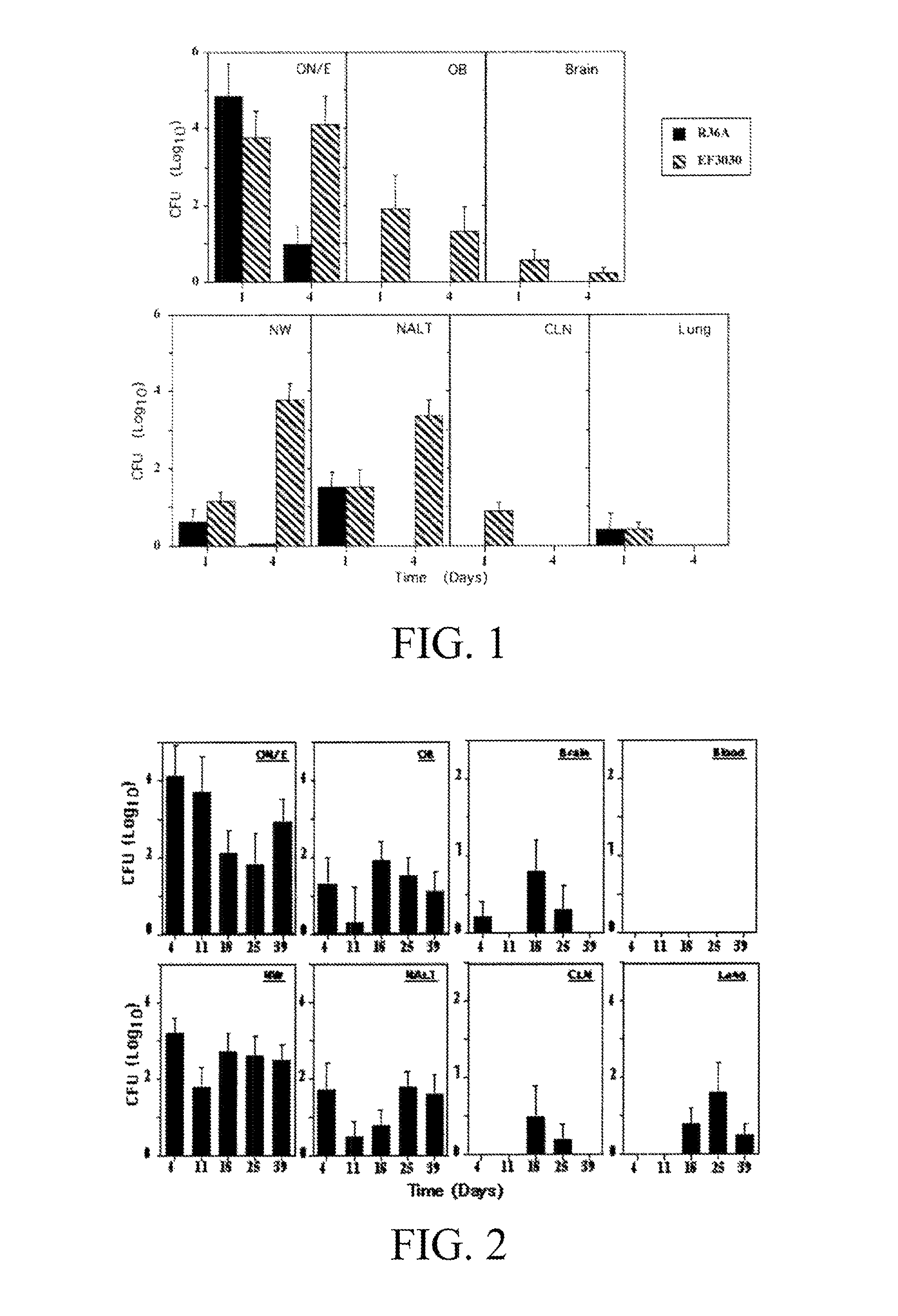
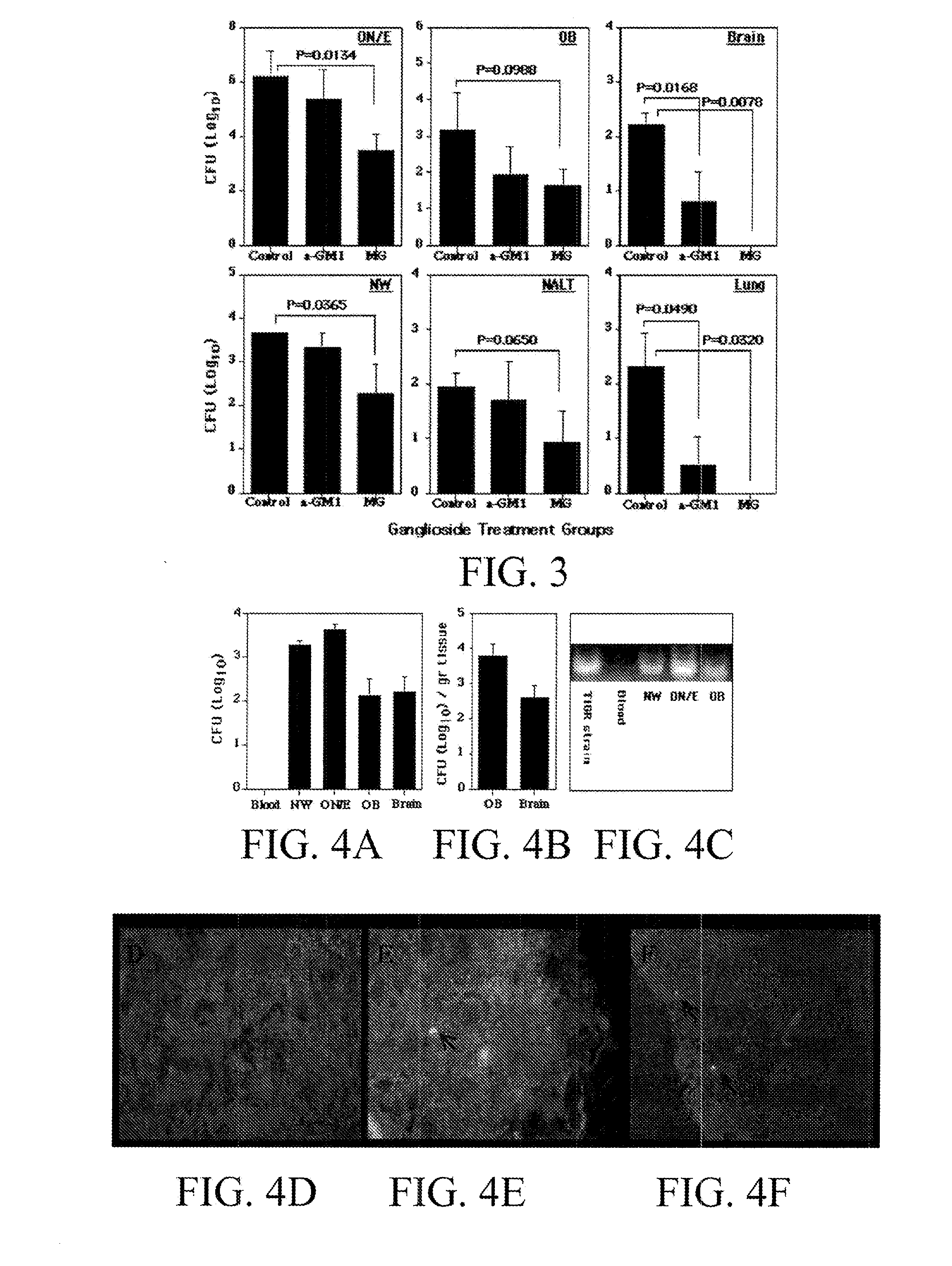
Provided herein are compositions designed to reduce or prevent bacterial infections (for example pneuomococcal infections), nasal carriage, nasal colonization, and central nervous system invasion. Provided herein is a composition comprising a pneumococcal neuraminidase, phosphocholine, pneumococcal teichoic acid, pneumococcal lipoteichoic acid, or an antigenic portion of either neuraminidase, phosphocholine, pneumococcal teichoic acid, pneumococcal lipoteichoic acid. Optionally, the composition can comprise any combination of a pneumococcal neuraminidase, a phosphocholine, a pneumococcal teichoic acid, a pneumococcal lipoteichoic acid or an antigenic portion of any one of these. Optionally the agents are detoxified. Further provided are methods of making and using the compositions disclosed herein. Specifically provided are methods of generating antibodies in a subject comprising administering to the subject an agent or composition taught herein. Also provided are methods of reducing or preventing nasal carriage or pneumococcal infection in a subject comprising administering to the subject a composition taught herein.
Owner:UAB RES FOUND
Chinese medicinal composition for treating chronic obstructive pulmonary disease
The invention discloses a Chinese medicinal composition for treating a chronic obstructive pulmonary disease in a stable period. The Chinese medicinal composition consists of five bulk pharmaceuticals including crude astragalus, Chinese magnoliavine fruit, epimedium herb, divaricate saposhnikovia root and honey-fried inula flower (decocted in a packet). As proved by pharmaceutical effect experiments, the Chinese medicinal composition has remarkable asthma-preventing, phlegm-eliminating and cough-relieving effects, and has certain suppressing effects on rat and mouse exudative inflammations; and as proved by in-vitro experiments, the Chinese medicinal composition has certain bacteriostasis effects on common respiratory tract pathogens, and has remarkable protecting effects on mice infected with staphylococcus aureus and pneumococcus. Meanwhile, as proved by clinical test researches, the Chinese medicinal composition has remarkable advantages on the aspects of asthma prevention, phlegm elimination and sign improvement.
Owner:李友林
Immunogenic compositions for use in pneumococcal vaccines
ActiveUS20190000952A1Raise the ratioIncrease OPA titerBacterial antigen ingredientsAntiinfectivesCoccidiaSalmonella serotype typhi
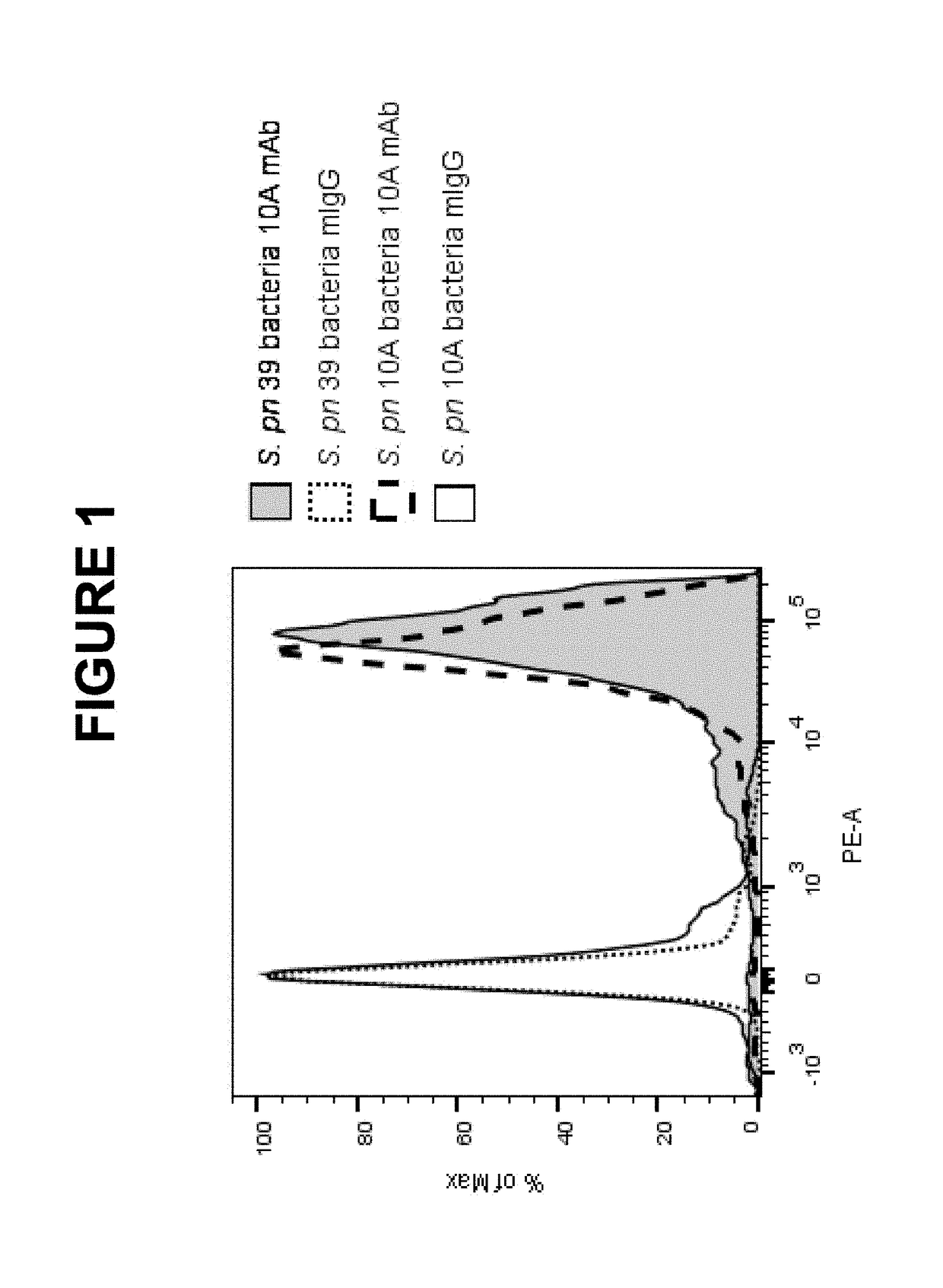
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
Immunogenic compositions for use in pneumococcal vaccines
An object of the present invention is to provide immunogenic compositions for protection against S. pneumoniae, in particular against S. pneumoniae serogroup 10A and 39, while limiting the number of conjugates. The present invention therefore relates to new immunogenic compositions for use in pneumococcal vaccines and to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said immunogenic compositions.
Owner:PFIZER INC
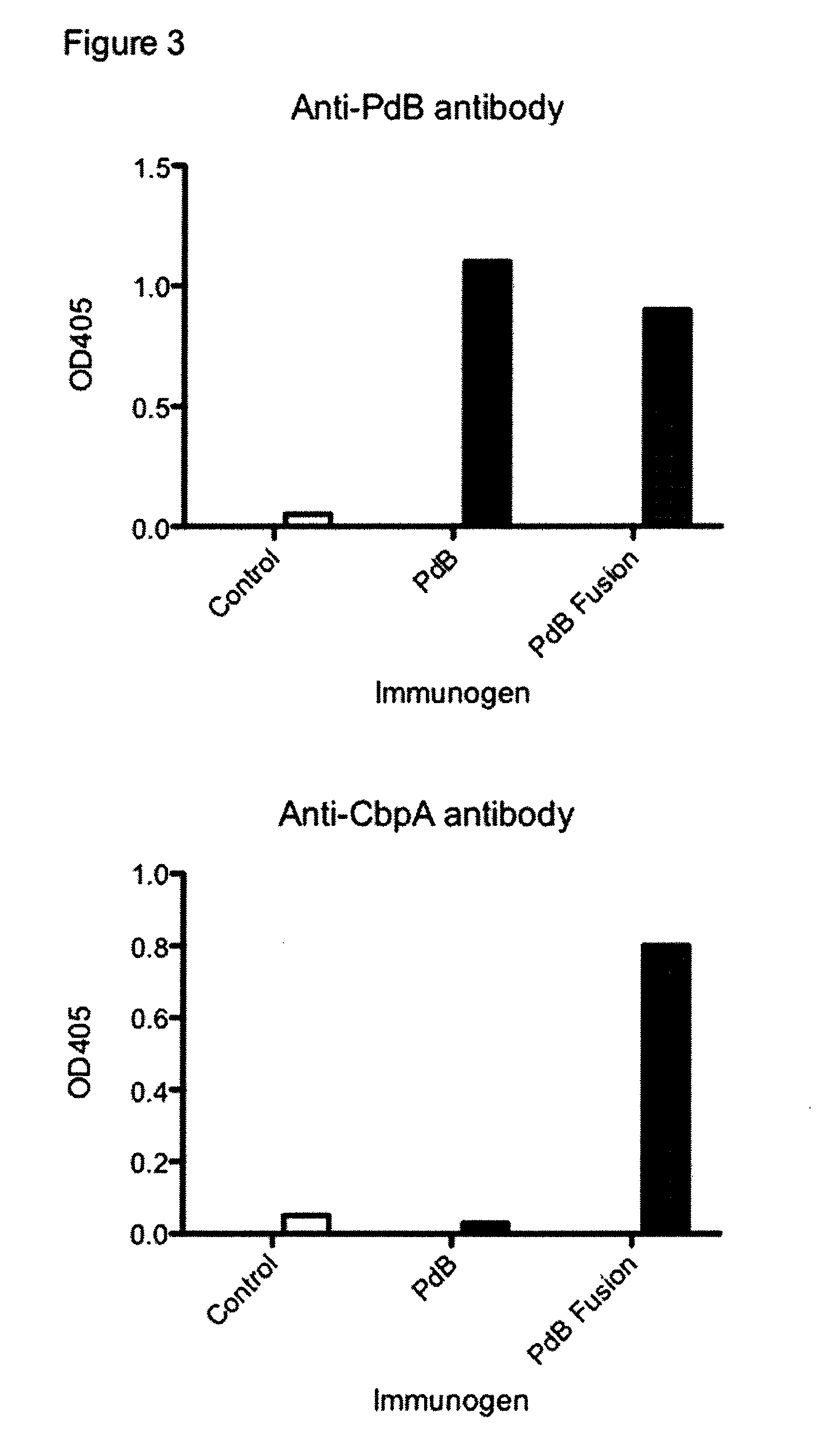
Methods and compositions employing immunogenic fusion proteins
Compositions and methods are provided for the prevention and treatment of bacterial infections, including pneumococal infections. Compositions provided herein comprise a variety of immunogenic fusion proteins, wherein at least one polypeptide component of a given fusion protein comprises a CbpA polypeptide and / or a cytolysoid polypeptide, or an active variant or fragment thereof. Methods are provided for the prevention and treatment of bacterial infections, including pneumococcal infections by employing the various immunogenic fusion proteins having at least one polypeptide component comprising a CbpA polypeptide and / or a cytolysoid polypeptide, or an active variant or fragment thereof.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
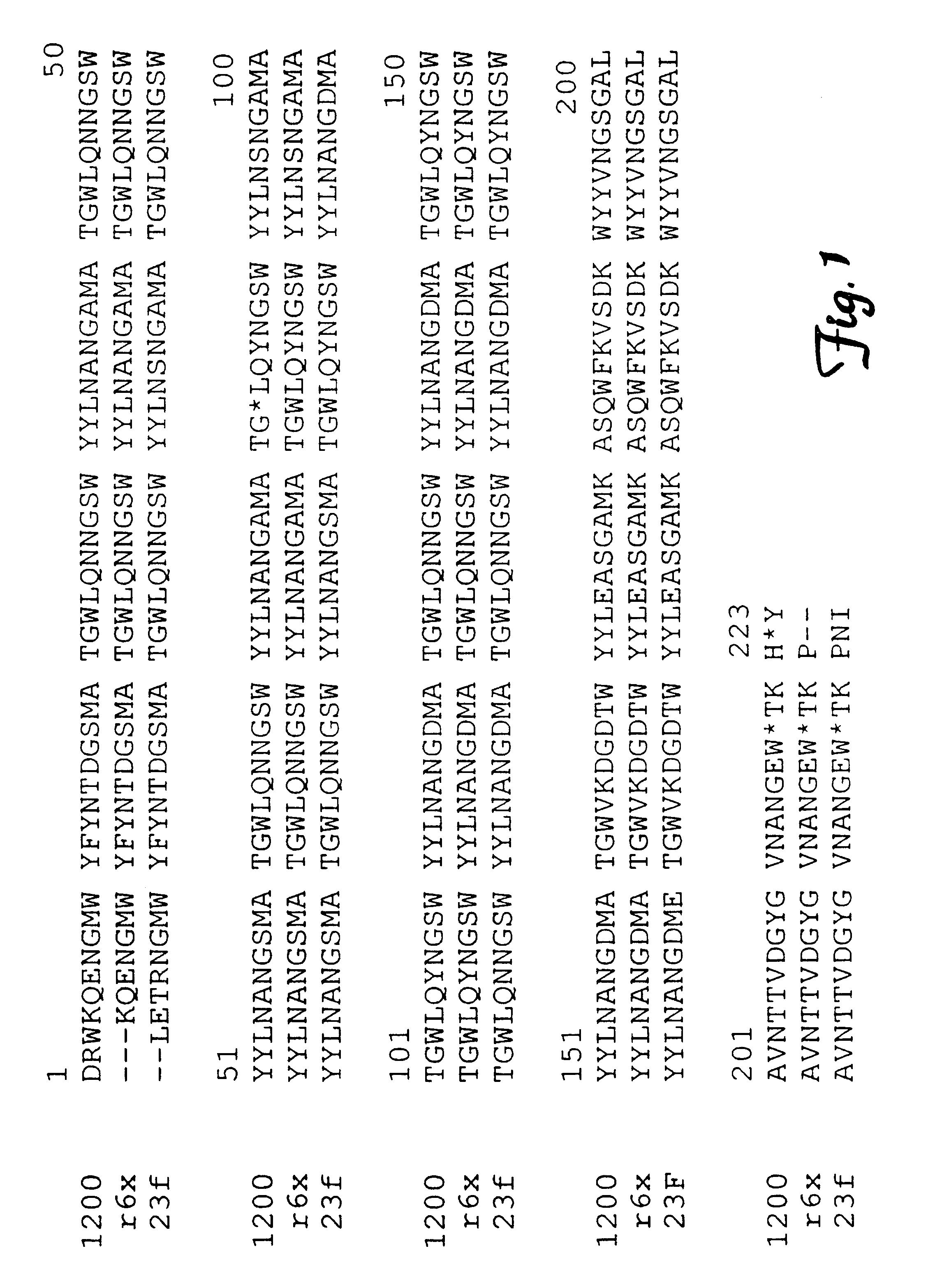
Streptococcus pneumonia autoenzyme protein and its protocaryon expression purification technique and vaccine
The invention discloses a method to separate and purify pneumonia streptococcus autolytic enzyme protein and protein vaccine to evoke effective protecting effect in molecular biology and genetic engineering technical domain, which is characterized by the following: cloning and transforming vaccine protein coding gene; constructing stability engineering bacteria; constituting stable high expression quantity protein separating and purifying system; evaluating protecting effect of animal experiment immunity; analyzing and evaluating protein physicochemical property; generating prevention function for respiratory tract se-producing germ; assuring antigen epitope of the protein; constructing recombinant antigen epitope with molecular clone technique; expressing in pronucleus expressing system.
Owner:SUN YAT SEN UNIV
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20200306357A1Bacterial antigen ingredientsPharmaceutical non-active ingredientsPrevnar 13Glycoconjugate
Owner:PFIZER INC
Novel immunogens and methods for discovery and screening thereof
ActiveUS20170028050A1Reduce or protect against pneumococcal colonizationReduce colonizationAntibacterial agentsBacterial antigen ingredientsBiological bodyCoccidia
The present application is generally directed to methods for identifying immunogens from organisms and pathogens, and in particular for identifying immunogens which when administered as vaccines elicit a cellular and / or humoral immune response. The present application is also directed to pneumococcal T-cell immunogens, and vaccine compositions comprising one or a combination of pneumococcal immunogens and methods for treating or preventing pneumococcal infections using the vaccines thereof. The present invention also encompasses use of the pneumococcal immunogens for diagnostic purposes to identify a subject with a pneumococcal infection.
Owner:CHILDRENS MEDICAL CENT CORP
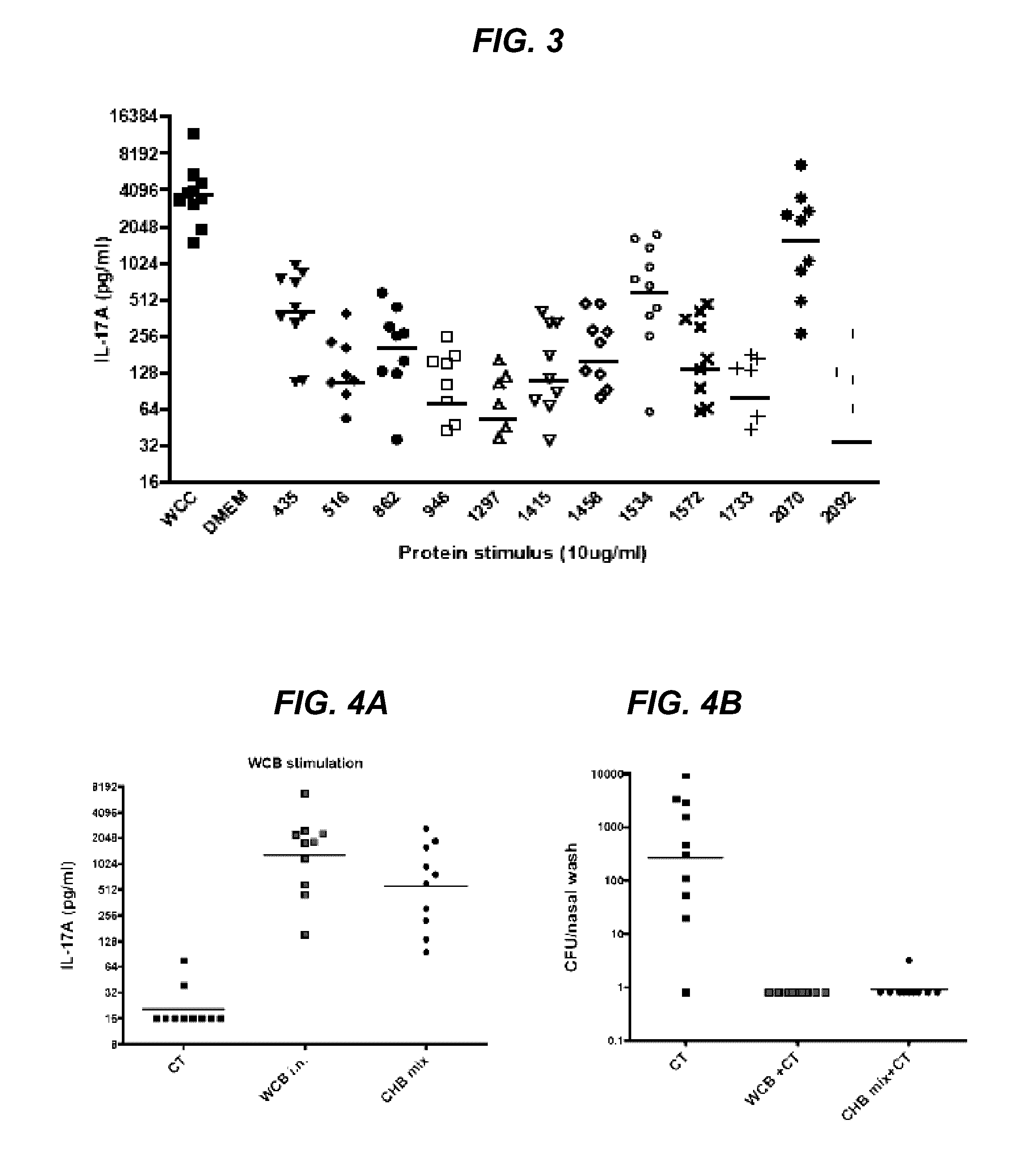
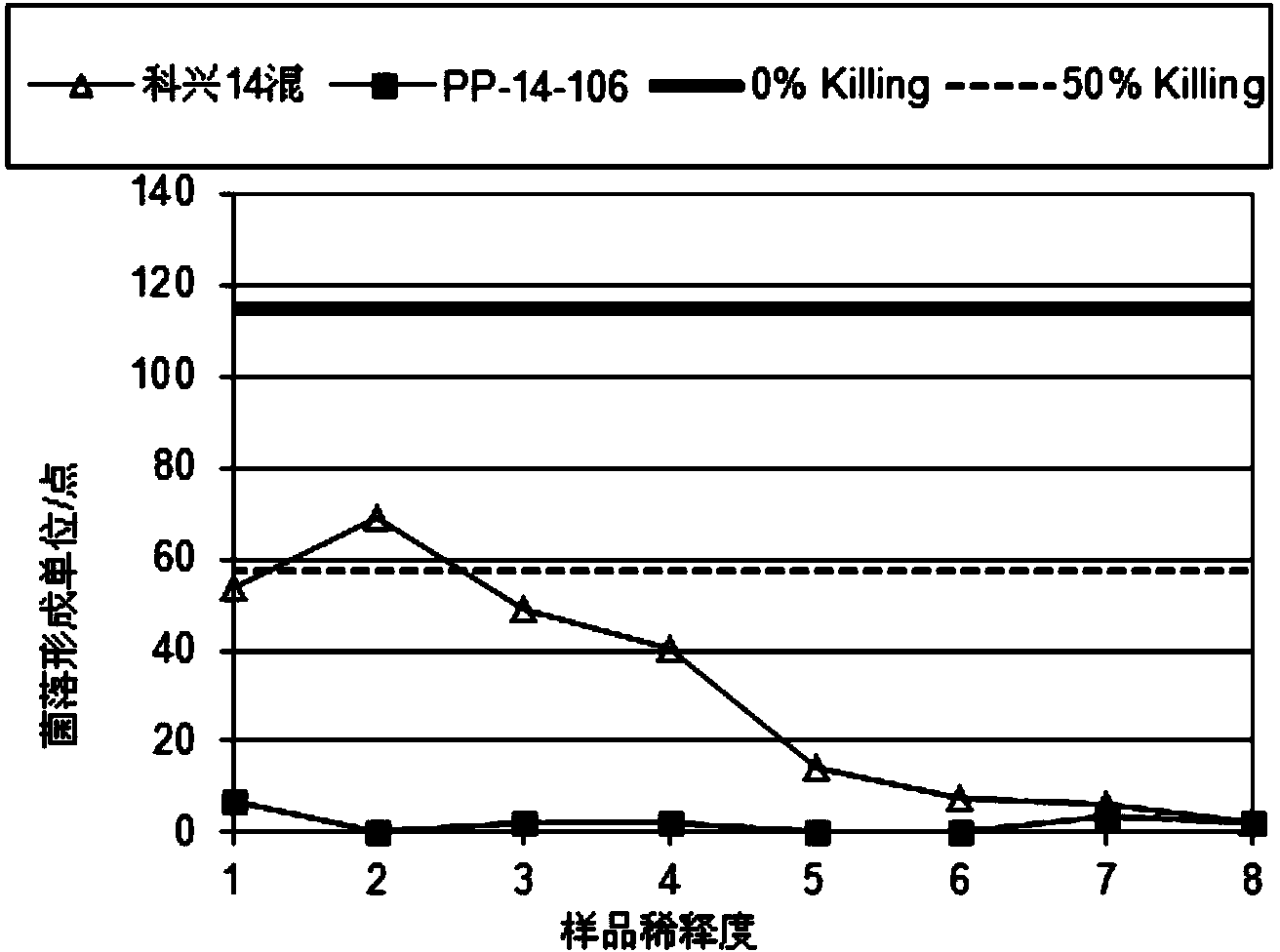
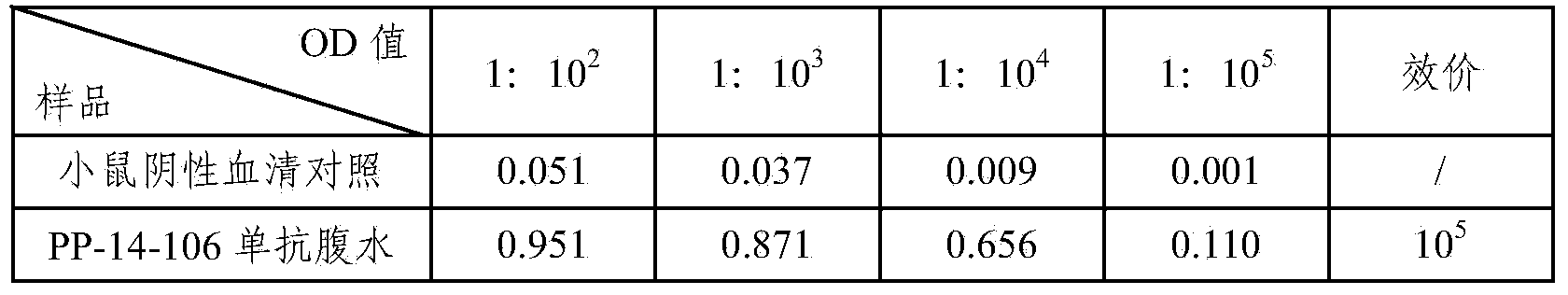
14 type pneumococcal neutralizing monoclonal antibody and application
InactiveCN104250635AImproving immunogenicityMeet the integration needsImmunoglobulins against bacteriaMicroorganism based processesBacteroidesPneumonia mrsa
The invention relates to an anti 14 type pneumococcal capsular polysaccharide neutralizing monoclonal antibody and a hybridoma cell strain for producing the anti 14 type pneumococcal capsular polysaccharide neutralizing monoclonal antibody, and the accession number of the hybridoma cell strain is CGMCC No.9455. The monoclonal antibody is high in titer and good in specificity, can be effective in vitro neutralization of 14 type pneumococcal infection, can effectively remove bacteria, has wide application prospect in the aspects of pneumonia diagnosis and treatment, can be applied to the pneumonia vaccine polysaccharide content detection, can be used for the polysaccharide content detection by enzyme linked immunosorbent assay, and breaks through the current detection methods.
Owner:SINOVAC RES & DEV
Synthetic Streptococcus pneumoniae vaccine
Compositions and methods for preventing and treating pneumococcal infections are provided. Compositions include novel polypeptides comprising an amino acid sequence corresponding to the R2i or R22 domain of CbpA or a consensus sequence of one of these domains, and variants and fragments thereof, wherein the polypeptide is stabilized in a desired conformation, particularly a loop conformation. The polypeptides of the invention may be engineered to comprise a first and a second cysteine residue, thereby resulting in the formation of a disulfide bond that stabilizes the polypeptide in the desired conformation. Alternatively, a polypeptide of the invention may be modified to create a synthetic linkage between a first and second amino acid residue present within the polypeptide, wherein the synthetic linkage stabilizes the polypeptide in the desired conformation. The polypeptides of the invention may further comprise an amino acid sequence for a T cell epitope. Compositions further include isolated nucleic acid molecules that encode the polypeptides of the invention, immunogenic compositions and vaccines comprising the disclosed polypeptides, and antibodies specific for these polypeptides.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Detoxified pneumococcal neuraminidase and uses thereof
InactiveUS20110104168A1Antibacterial agentsBacterial antigen ingredientsNeuraminidaseNasal colonization
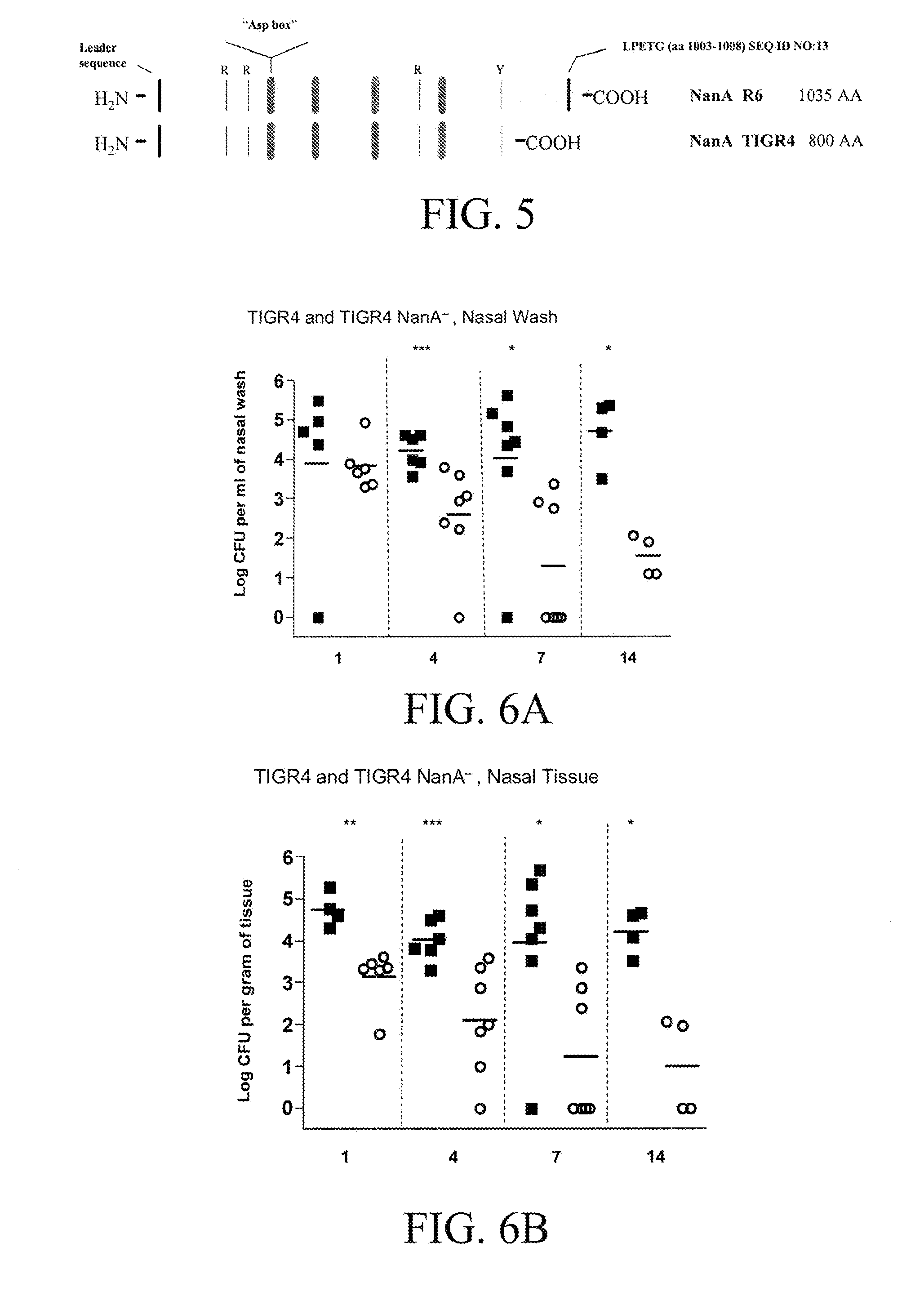
Provided herein are compositions designed to reduce or prevent pneuomococcal infections, nasal carriage, nasal colonization, and central nervous system invasion. Provided herein is a composition comprising a polypeptide comprising the amino acid sequence of SEQ ID NO: 19 or a variant thereof that can elicit an anti-neuraminidase immune response. Further provided are methods of making and using the compositions disclosed herein. Specifically provided are methods of generating antibodies in a subject comprising administering to the subject an agent or composition taught herein. Also provided are methods of reducing or preventing nasal carriage or pneumococcal infection in a subject comprising administering to the subject a composition taught herein.
Owner:UAB RES FOUND
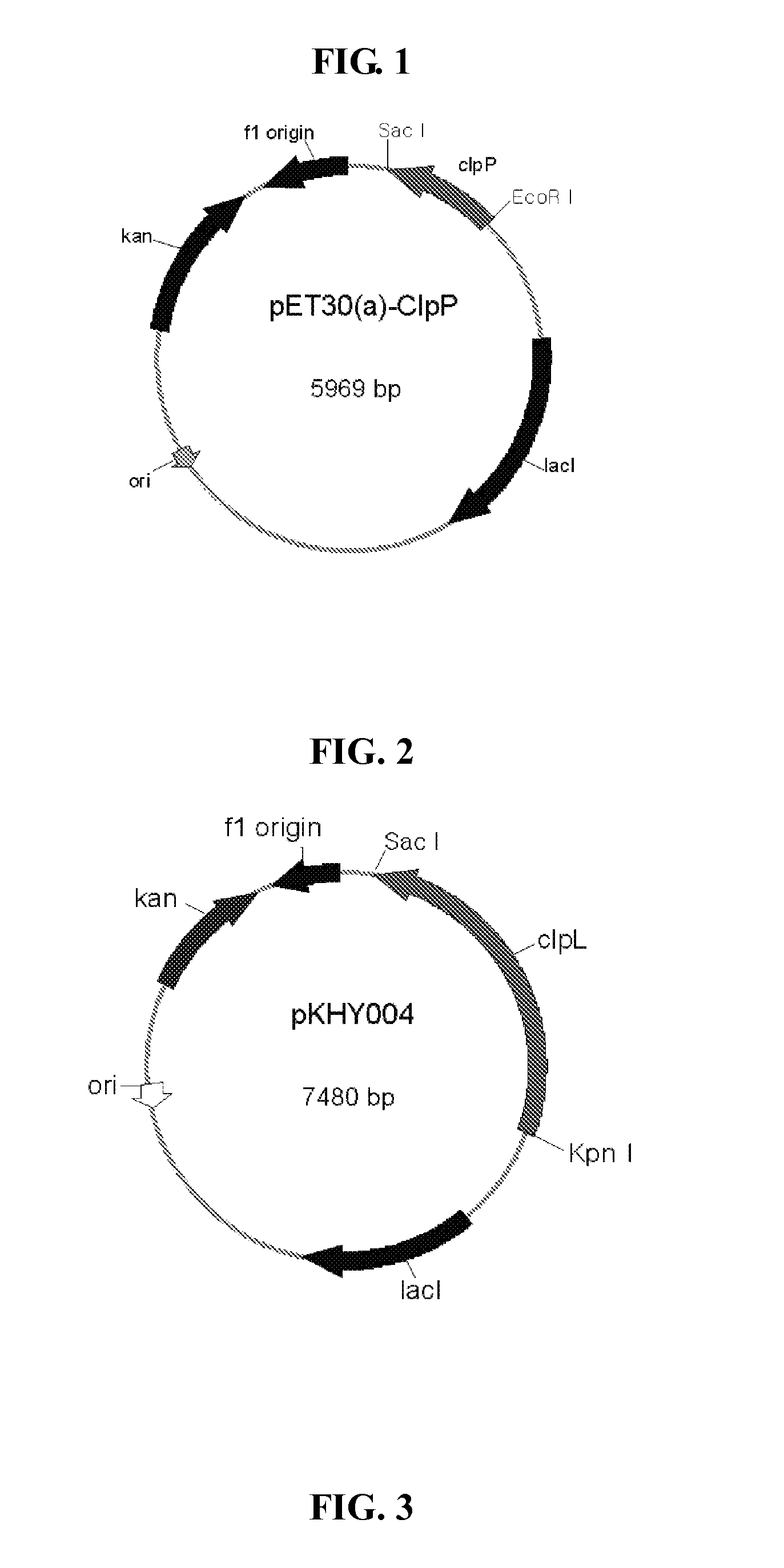
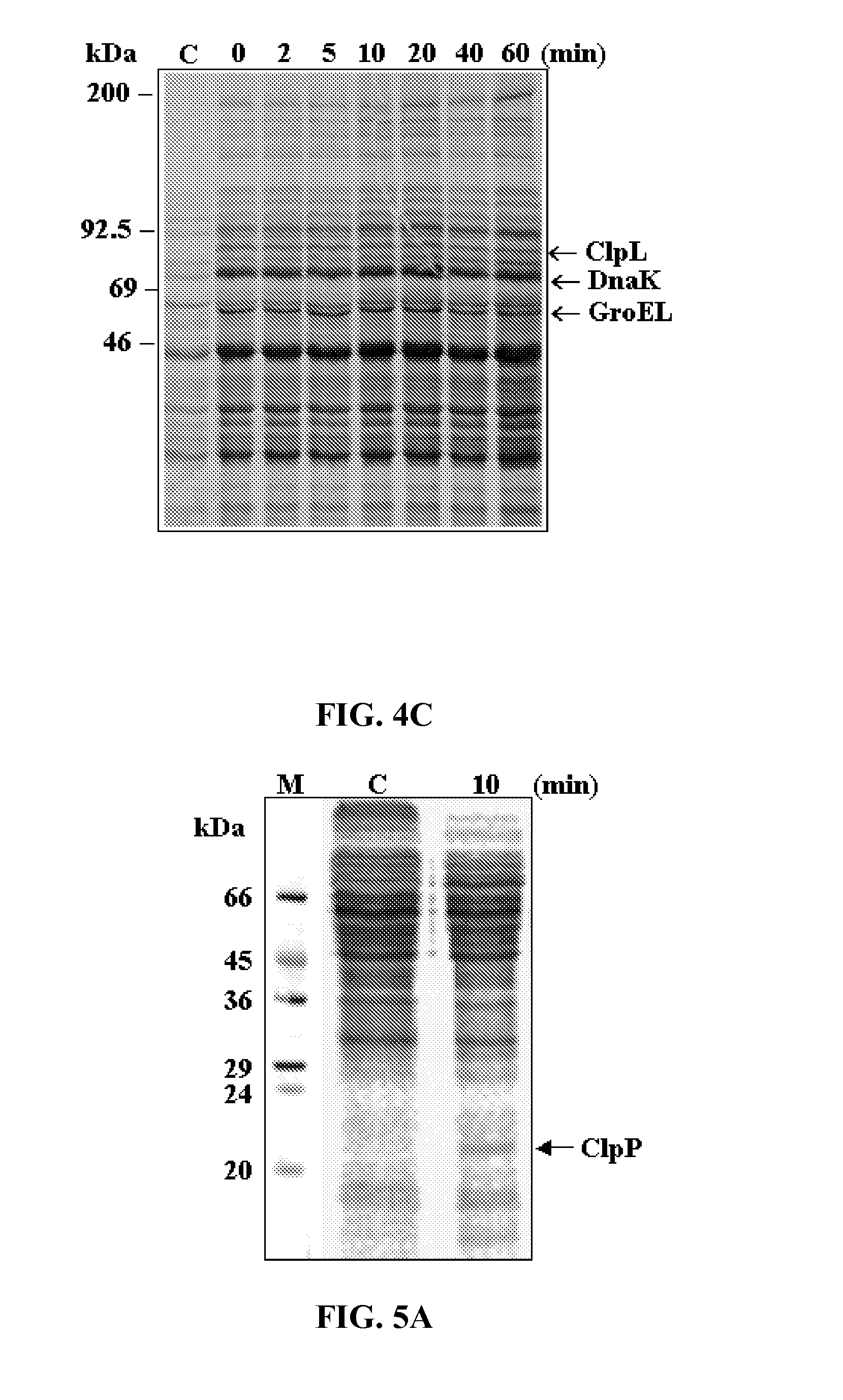
Vaccine comprising recombinant clpp protein of streptococcus pneumoniae
ActiveUS20110129501A1Increase mRNA expressionIncrease ratingsAntibacterial agentsBacterial antigen ingredientsSynechococcusStreptococcus mitis
A method for immunizing a human or animal against pneumococcal infections, comprising by administering a vaccine comprising a purified recombinant caseinolytic protease P (ClpP) protein of S. pneumoniae in an immunologically effective amount to the human or animal.
Owner:SUNGKYUNKWAN UNIVERSITY +1
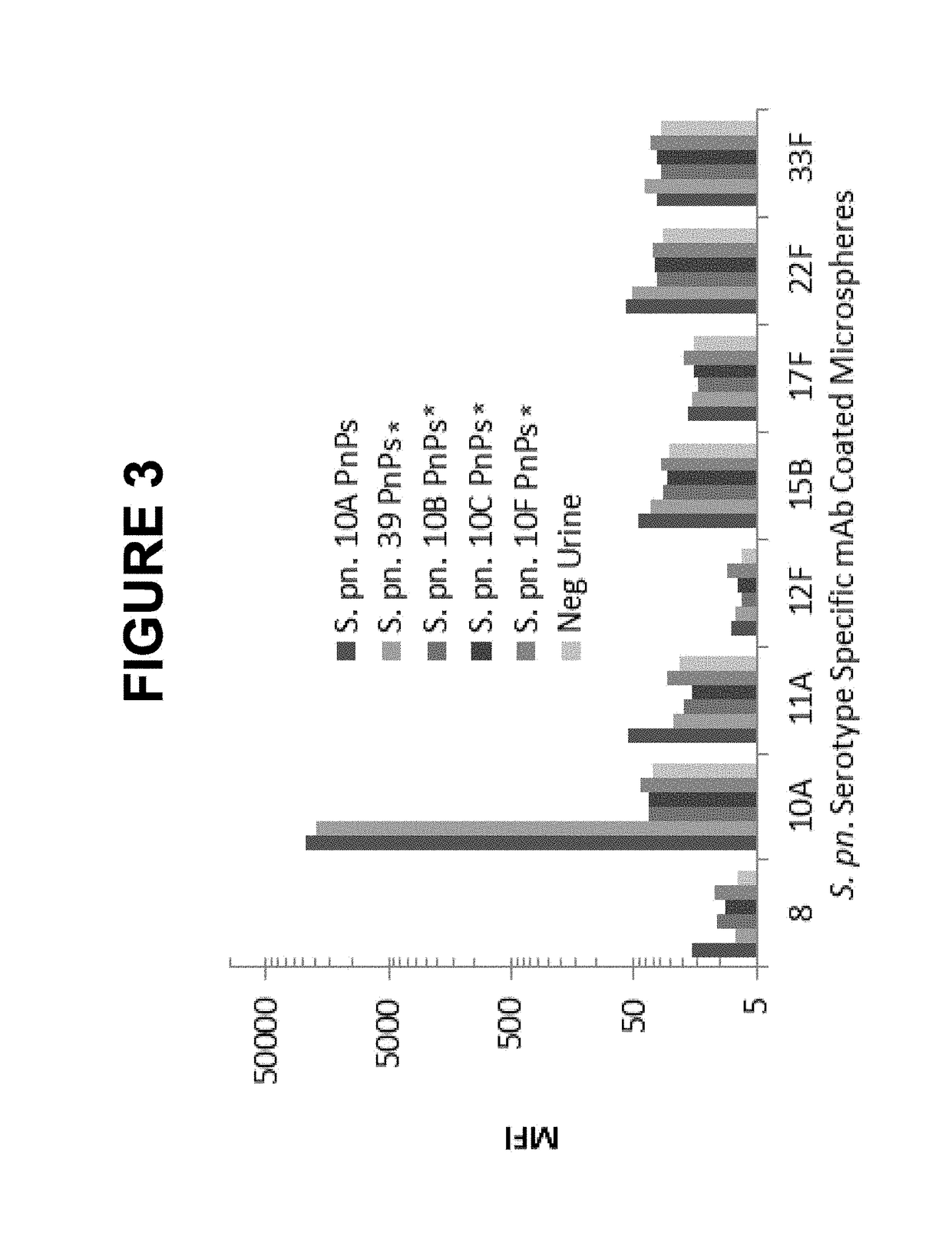
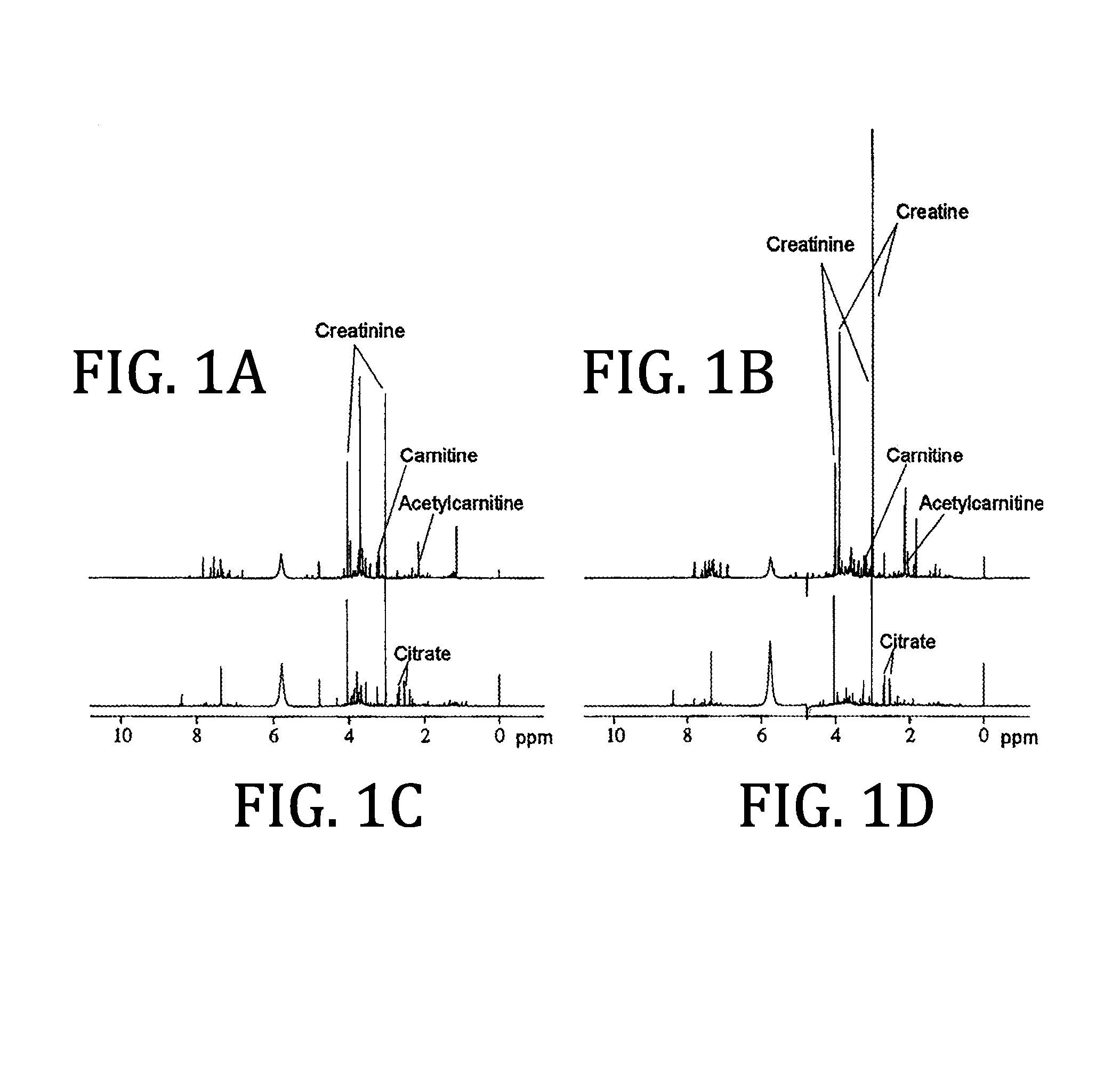
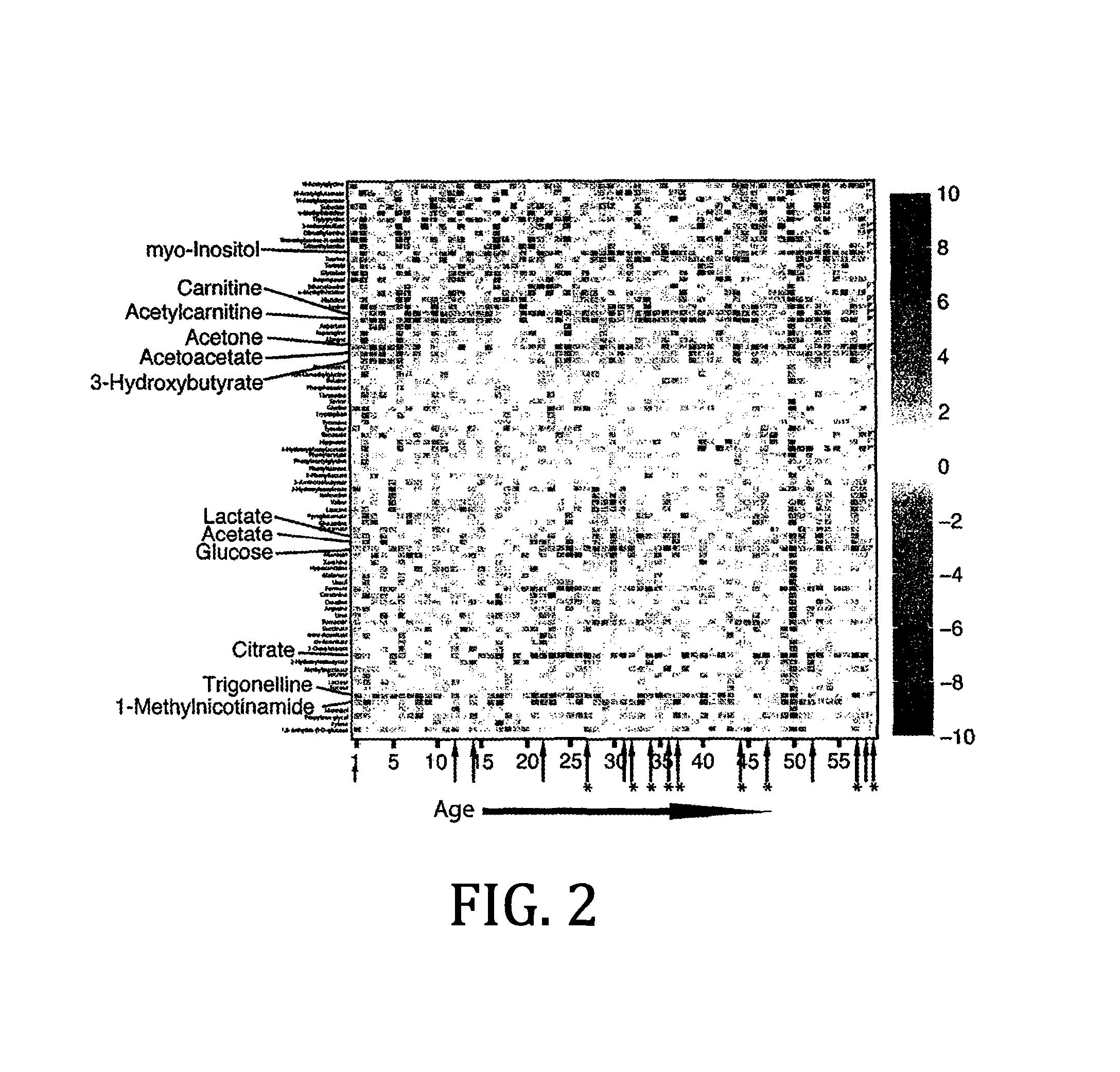
Urine based detection of a disease state caused by a pneumococcal infection
There is provided a method of diagnosing a disease state in a subject. The disease state is caused or effected by pneumococcal infection. The method includes obtaining a biological test sample from the subject. The biological sample includes at least one metabolite selected from the group consisting of citrate, trigonelline, acetylcarnitine, acetone, myo-inositol, 3-hydroxybutyrate, glucose and carnitine. A respective concentration of each one of the at least one metabolite is compared with a predetermined concentration value associated with a corresponding one of the at least one metabolite. The predetermined concentration value is indicative of the disease state. For example, the biological sample includes any one of citrate, trigonelline, acetylcamitine, acetone, myoinositol, 3-hydroxybutyrate, glucose and carnitine. As a further example, the biological samples include any combination of citrate, trigonelline, acetylcamitine, acetone, myo-inositol, 3-hydroxybutyrate, glucose and carnitine. As a further example, the biological sample includes each one of citrate, trigonelline, acetylcamitine, acetone, myoinositol, 3-hydroxybutyrate, glucose and carnitine.
Owner:SLUPSKY CAROLYN
Pyrrole derivatives
Owner:UNIVERSITY OF LEICESTER
Novel immunogens and methods for discovery and screening thereof
InactiveUS20130039947A1Reduce or protect against pneumococcal colonizationReduce colonizationAntibacterial agentsAntimycoticsT cell immunityHumoral immunity
The present application is generally directed to methods for identifying immunogens from organisms and pathogens, and in particular for identifying immunogens which when administered as vaccines elicit a cellular and / or humoral immune response. The present application is also directed to pneumococcal T-cell immunogens, and vaccine compositions comprising one or a combination of pneumococcal immunogens and methods for treating or preventing pneumococcal infections using the vaccines thereof. The present invention also encompasses use of the pneumococcal immunogens for diagnostic purposes to identify a subject with a pneumococcal infection.
Owner:CHILDRENS MEDICAL CENT CORP
Detoxified pneumococcal neuraminidase and uses thereof
Provided herein are compositions designed to reduce or prevent pneuomococcal infections, nasal carriage, nasal colonization, and central nervous system invasion. Provided herein is a composition comprising a polypeptide comprising the amino acid sequence of SEQ ID NO: 19 or a variant thereof that can elicit an anti-neuraminidase immune response. Further provided are methods of making and using the compositions disclosed herein. Specifically provided are methods of generating antibodies in a subject comprising administering to the subject an agent or composition taught herein. Also provided are methods of reducing or preventing nasal carriage or pneumococcal infection in a subject comprising administering to the subject a composition taught herein.
Owner:UAB RES FOUND
Use of a compound and its derivatives for treating pneumococcal infectious diseases
ActiveCN104224810BInhibition of hydrolysis reactionGrowth inhibitionAntibacterial agentsBoron compound active ingredientsPathogenic microorganismHuman cell
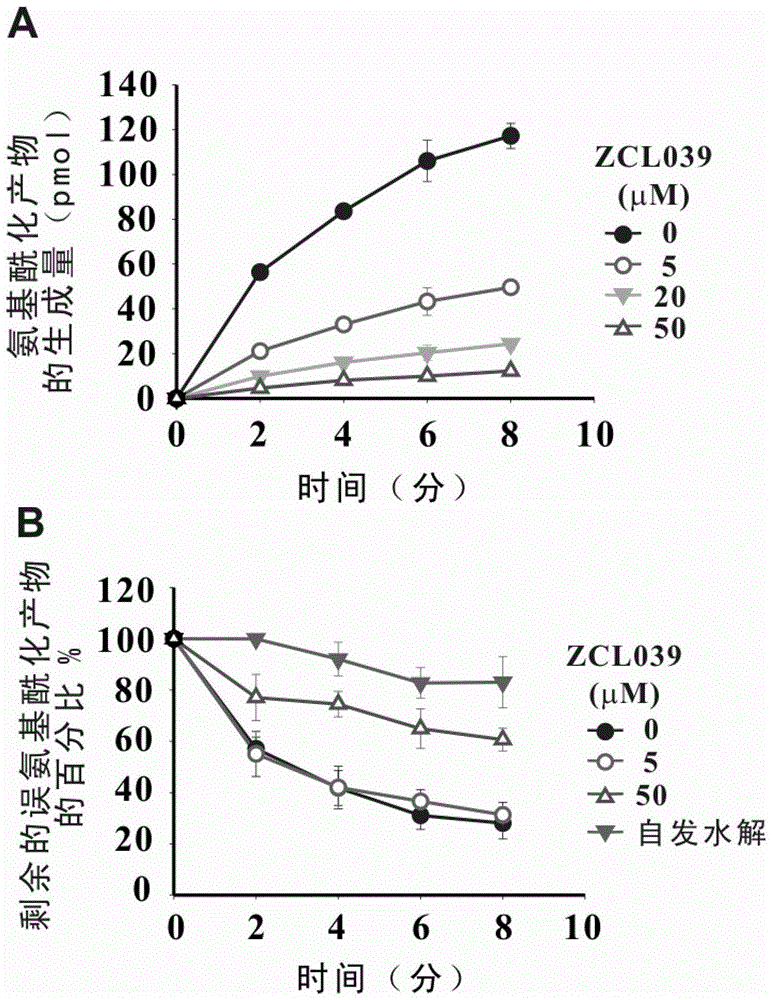
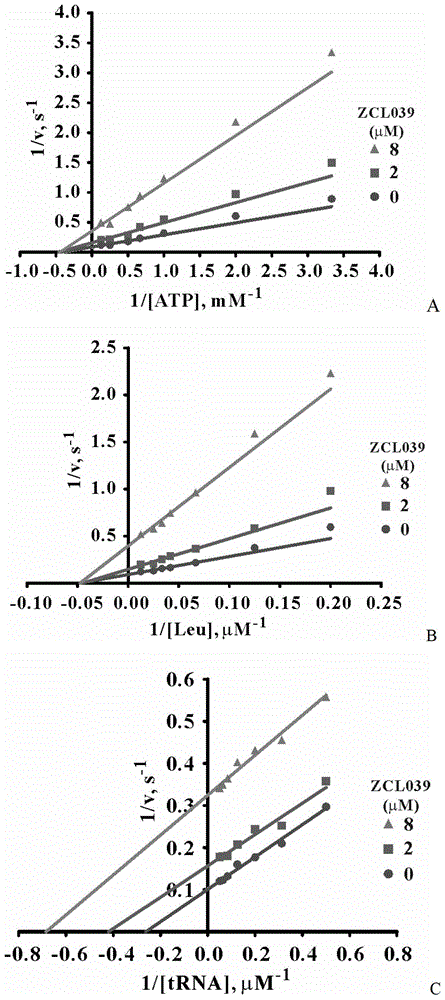
The invention relates to the field of pharmaceutical chemistry, and particularly relates to a novel compound for treating pneumococcal infectious diseases, and use of the compound. According to the compound disclosed by the invention, the structural general formula is as shown in the formula I. The compound disclosed by the invention, especially ZCL039 can be applied to preparing a leucyl-tRNA synthetase inhibitor, and medicines for treating pneumococcal infectious diseases, and can be used for inhibiting or killing pathogenic microorganisms such as pneumococcus and the like. The compound disclosed by the invention has the advantage of activity of resisting pneumococcus, has a strong function of inhibiting SpLeuRS, is low in toxicity on human cells, and provides a new path for treatment of the pneumococcal infectious diseases.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Anti-pneumococcal hyperimmune globulin for the treatment and prevention of pneumococcal infection
ActiveUS10259865B2Reduce morbidityImprove the level ofAntibacterial agentsSerum immunoglobulinsSinusitisPneumococcal antibodies
The present invention relates to compositions and methods for the treatment of infection caused by Streptococcus pneumonia. In particular, the invention provides human hyperimmune globulin and compositions thereof for preventing or treating pneumococcal infection. The invention provides methods of producing hyperimmune globulin containing high titers of opsonophagocytic anti-pneumococcal antibodies, compositions containing same, and methods of using the compositions for the prevention and treatment of pneumococcal infection. The invention further provides methods of preventing or treating pneumococcal infection (e.g., upper respiratory infections (e.g., bronchitis, otitis, sinusitis, etc.)) in immunocompromised subjects via administration of hyperimmune globulin compositions of the invention (e.g., containing a high titer of opsonophagocytic anti-pneumococcal antibodies) to immunocompromised subjects.
Owner:ADMA BIOMANUFACTURING LLC
Vaccine for preventing pneumococus infection
The invention relates to the field of biology, more specifically to the field of immunology and microbiology. The invention further relates to the field of vaccines against microbial infections and especially bacterial vaccines, in particular to pneumococcal vaccines. More in particular, the invention relates to means and methods to identify, select and isolate a vaccine component for passive and / or active immunisation against a microorganism that can be killed by opsonophagocytic cells. <??>The invention relates to a method to identify an opsonophagocytosis inducing antigen as a vaccine component for immunisation against a microorganism. The invention describes three pneumococcal proteins SlrA, IgA1 proteinase, and PsaA, and their use as a vaccine component with or without PpmA. <??>The invention also discloses the use of antibodies against said proteins for passive immunisation.
Owner:MUCOSIS
Virulence factors of Streptococcus pnuemoniae
ActiveUS10485860B2Bacterial antigen ingredientsSnake antigen ingredientsVirulent characteristicsCoccidia
The present invention provides proteins / genes, which are essential for survival, and consequently, for virulence of Streptococcus pneumoniae in vivo, and thus are ideal vaccine candidates for a vaccine preparation against pneumococcal infection. Further, also antibodies against said protein(s) are included in the invention.
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com