Cysteine engineered antibodies and conjugates
a technology of engineered antibodies and conjugates, applied in the field of antibodies, can solve the problems of potential problems such as the inability to engineer in cysteine thiol groups by the mutation of various amino acid residues of a protein to cysteine amino acids, and the inability to separate analytical and preparative methods,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Biotinylated ThioFab Phage
[0292]ThioFab-phage (5×1012 phage particles) were reacted with 150 fold excess of biotin-PEO-maleimide ((+)-biotinyl-3-maleimidopropionamidyl-3,6-dioxaoctainediamine, Oda et al (2001) Nature Biotechnology 19:379-382, Pierce Biotechnology, Inc.) for 3 hours at room temperature. Excess biotin-PEO-maleimide was removed from biotin-conjugated phage by repeated PEG precipitations (3-4 times). Other commercially available biotinylation reagents with electrophilic groups which are reactive with cysteine thiol groups may be used, including Biotin-BMCC, PEO-Iodoacetyl Biotin, Iodoacetyl-LC-Biotin, and Biotin-HPDP (Pierce Biotechnology, Inc.), and Nα-(3-maleimidylpropionyl)biocytin (MPB, Molecular Probes, Eugene, Oreg.). Other commercial sources for biotinylation, bifunctional and multifunctional linker reagents include Molecular Probes, Eugene, Oreg., and Sigma, St. Louis, Mo.
example 2
PHESELECTOR Assay
[0293]Bovine serum albumin (BSA), erbB2 extracellular domain (HER2) and streptavidin (100 μl of 2 μg / ml) were separately coated on Maxisorp 96 well plates. After blocking with 0.5% Tween-20 (in PBS), biotinylated and non-biotinylated hu4D5Fabv8-ThioFab-Phage (2×1010 phage particles) were incubated for 1 hour at room temperature followed by incubation with horseradish peroxidase (HRP) labeled secondary antibody (anti-M13 phage coat protein, pVIII protein antibody). FIG. 8 illustrates the PHESELECTOR Assay by a schematic representation depicting the binding of Fab or ThioFab to HER2 (top) and biotinylated ThioFab to streptavidin (bottom).
[0294]Standard HRP reaction was carried out and the absorbance was measured at 450 nm. Thiol reactivity was measured by calculating the ratio between OD450 for streptavidin / OD450 for HER2. A thiol reactivity value of 1 indicates complete biotinylation of the cysteine thiol. In the case of Fab protein binding measurements, hu4D5Fabv8 (...
example 3a
Expression and Purification of ThioFabs
[0295]ThioFabs were expressed upon induction in 34B8, a non-suppressor E. coli strain (Baca et al (1997) Journal Biological Chemistry 272(16):10678-84). The harvested cell pellet was resuspended in PBS (phosphate buffered saline), total cell lysis was performed by passing through a microfluidizer and the ThioFabs were purified by affinity chromatography with protein G SEPHAROSE™ (Amersham).
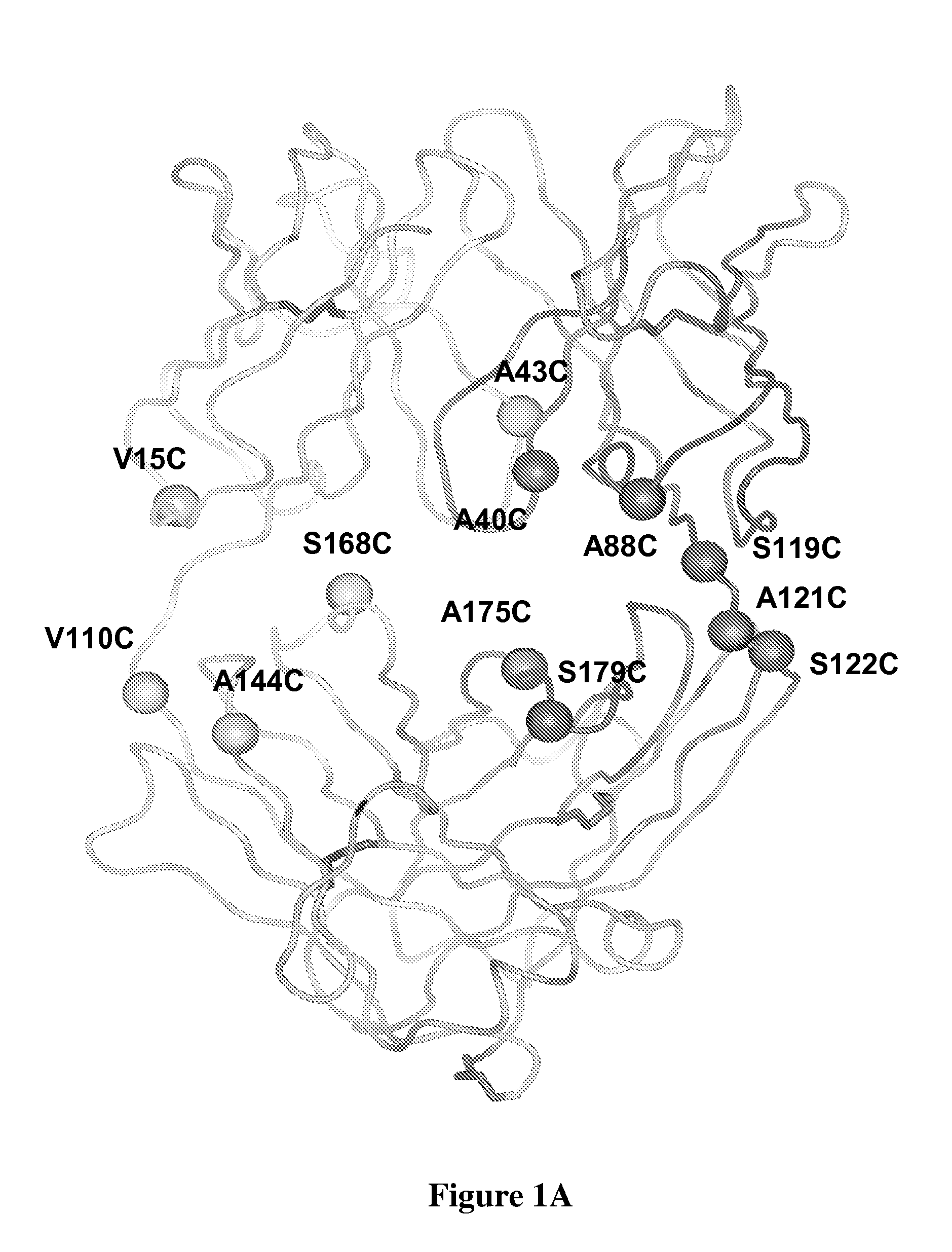
[0296]ThioFabs L-V15C, L-V110C, H-A88C, and H-A121C were expressed and purified by Protein-G SEPHAROSE™ column chromatography. Oligomeric-Fab was present in fractions 26 to 30, and most of the monomeric form was in fractions 31-34. Fractions consisting of the monomeric form were pooled and analyzed by SDS-PAGE along with wild type hu4D5Fabv8 and analyzed on SDS-PAGE gel in reducing (with DTT or BME) and non-reducing (without DTT or BME) conditions. Gel filtration fractions of A121C-ThioFab were analyzed on non-reducing SDS-PAGE.
[0297]ThioFabs were conjugated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Mass spec protein sequencing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com