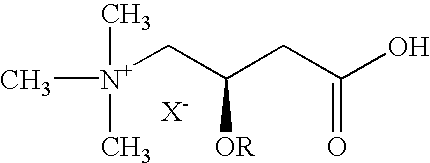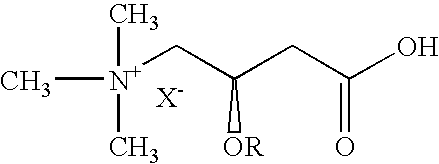Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
81 results about "Vinca alkaloid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus (basionym Vinca rosea) and other vinca plants. They block beta-tubulin polymerization in a dividing cell.
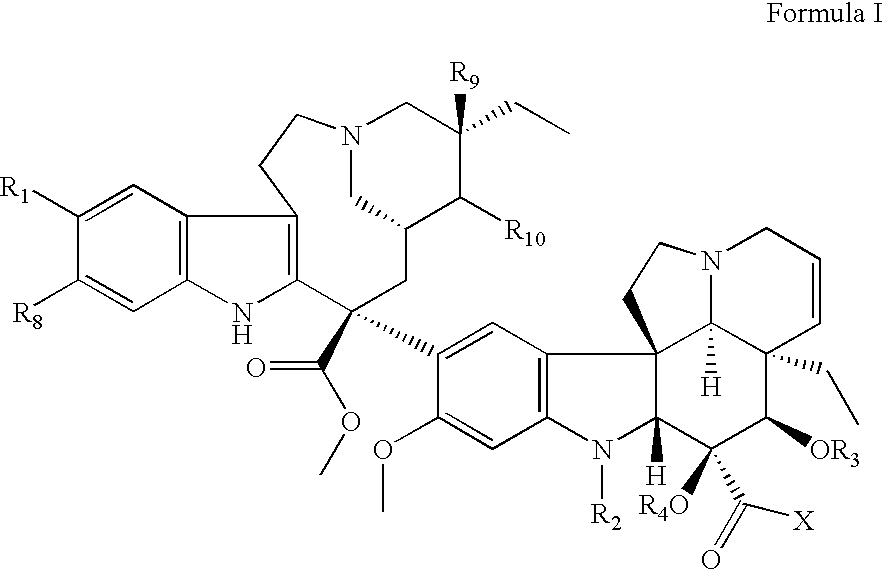
Ligand Conjugates of Vinca Alkaloids, Analogs, and Derivatives
Described herein are compounds, pharmaceutical compositions and methods for treating pathogenic cell populations in a patient. The compounds described herein include conjugates of cytotoxic drugs and vitamin receptor binding ligands. The conjugates also include a linker that is formed from one or more spacer linkers, heteroatom linkers, and releasable linkers.
Owner:ENDOCTYE INC
Enhanced B cell cytotoxicity of CDIM binding antibody
InactiveUS20050112130A1Good curative effectStrong cytotoxicityOrganic active ingredientsPeptide/protein ingredientsAutoimmune conditionCytotoxicity
Formulations and methods of treating human patients suffering from a condition characterized by lymphoid cancer, autoimmune disease or B cell hyperproliferation are disclosed, the treatment comprising administering (1) a cytotoxic amount of an antibody having specific binding for CDIM epitopes on a B cell, and (2) a cytotoxic agent, including a chemotherapeutic agent, radioactive isotope, cytotoxic antibody, immunoconjugate, ligand conjugate, immunosuppressant, cell growth regulator and / or inhibitor, toxin, or mixtures thereof, including agents that disrupt the cytoskeleton of B cells, particularly vinca alkaloids or colchicine.
Owner:PALIGEN INC +2
Compositions for delivering highly water soluble drugs
InactiveUS20060008480A1Increase in sizeOrganic active ingredientsSolution deliveryMedicineWater soluble
Owner:MAST THERAPEUTICS
Weight control compositions and methods
InactiveUS20050025844A1Decreasing body fatIncreasing and maintaining lean body massBiocideUnknown materialsN-MethyltyramineReceptor antagonist
The present invention provides compositions and methods that assist in providing weight control. Compositions comprise Caffeine, an adrenergic amine (e.g. synephrine, hordenine, octopamine, tyramine and N-methyltyramine,) forskolin, Guggulsterones, an alpha-2 receptor antagonist (e.g. Yohimbine) and a vinca alkaloid (e.g. vinpocetine.) Black pepper extract may be added as well in various alternative embodiments. Methods utilizing administration of nutrient compositions are disclosed as well.
Owner:SELLO AZUL
Stabilized oil-in-water emulsion of vinca alkaloids for vein and production thereof
InactiveCN1679576ALow toxicityLess irritatingOrganic active ingredientsEmulsion deliveryAdditive ingredientVinorelbine
A stable oil-in-water emulsion of vinorelbine for intravenous injection is prepared from vinorelbine, the oil for injection, emulsifier, and the water for injection. Its advantages are high tageting function, low poison, and high safety.
Owner:JIANGSU QINGJIANG PHARMA
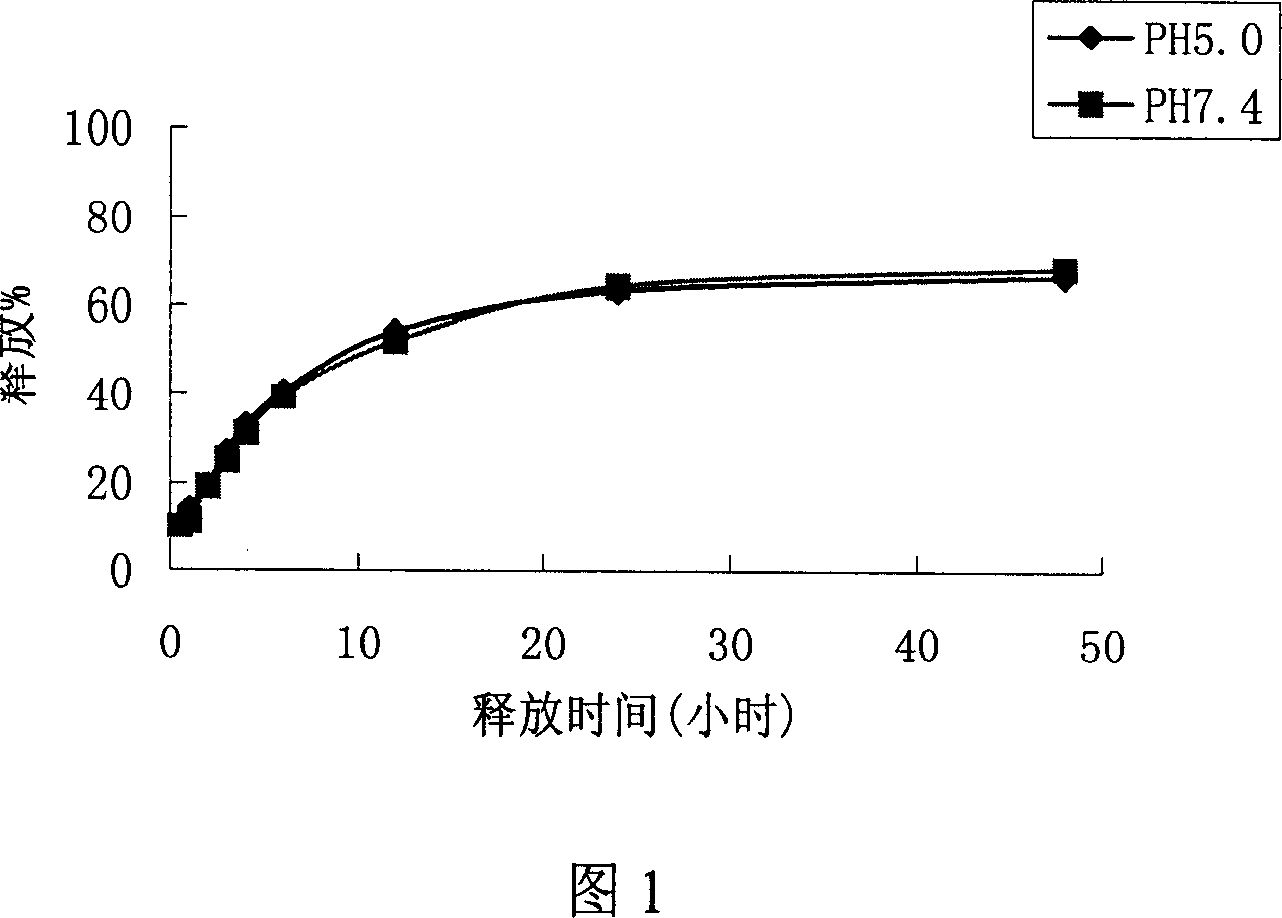
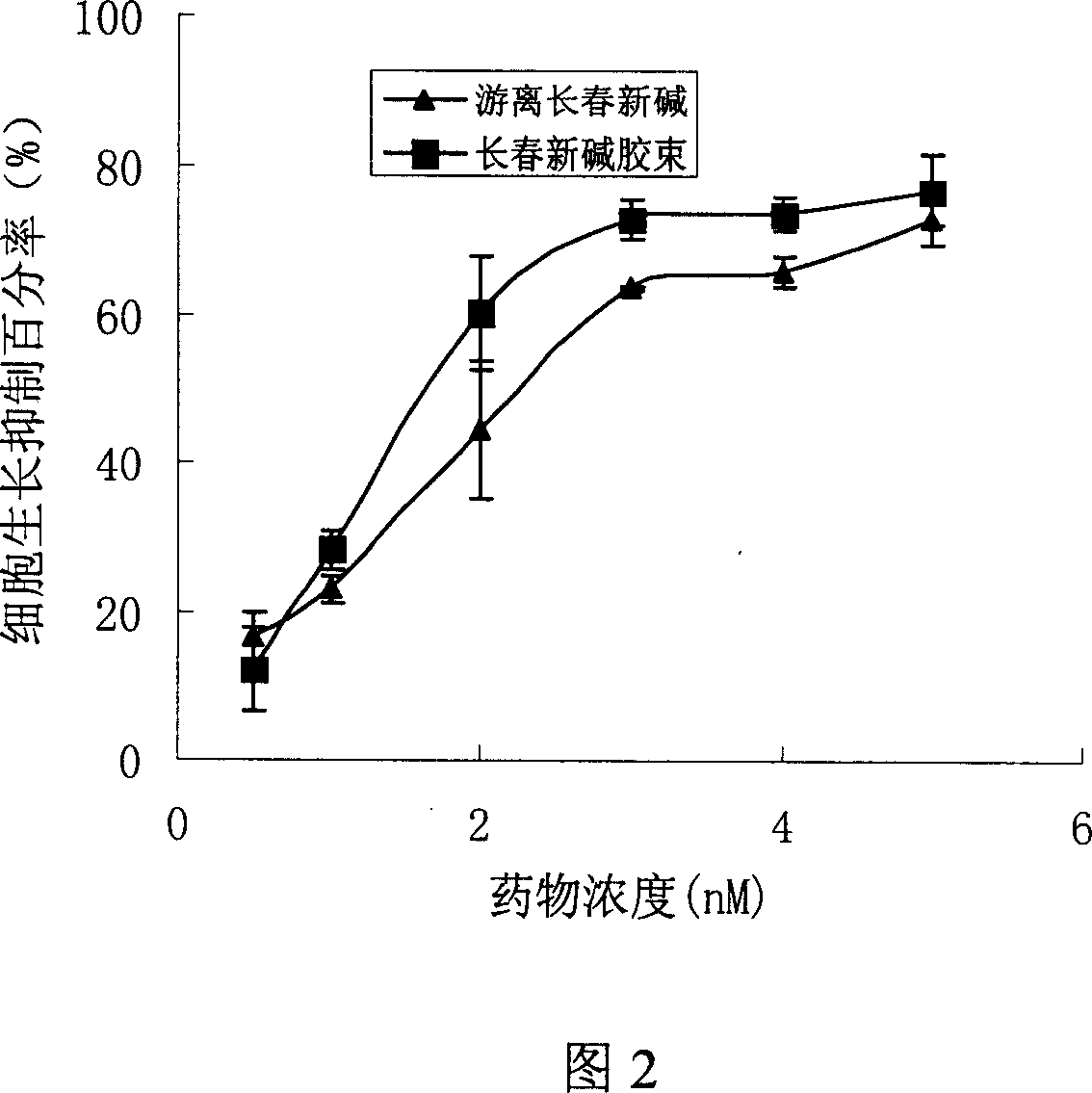
Nano micelle preparation of Catharanthus roseus alkaloids antineoplastic drugs with coating of phospholipid derived from polyethylene glycol
The invention provides intravenous nanomicelle agents of vinca alkaloids antitumor drug, it contains an effective dose for treating of vinca alkaloids antitumor drug (vinblastine and vincristine or vindesine), macrogol derivatization phospholipid, and the pharmaceutical acceptable adjuvants. Its preparation is to pack the drug in the formative nanomicelle agents, prepare and make the intravenous nanomicelle agents of vinca alkaloids antitumor drug. Vinca alkaloids antitumor drug and macrogol derivatization phospholipid form into a very uniform size nanomicelle. In micelles, polyethylene glycol molecule and hydrophobic core for drug packing form a hydrophilicitious inhibitory coating, avoid the drugs contact with the protein such as enzymes in the blood and identified and phagocytized by the endothelial system in vivo, phagocytosis, the cycle time of micellar in vivo is extended. In addition, the micellar drug also increases the storage stability and the effect on the tumor of the drug and reduces drug toxicity.
Owner:BEIJING DEKERUI MEDICAL TECH
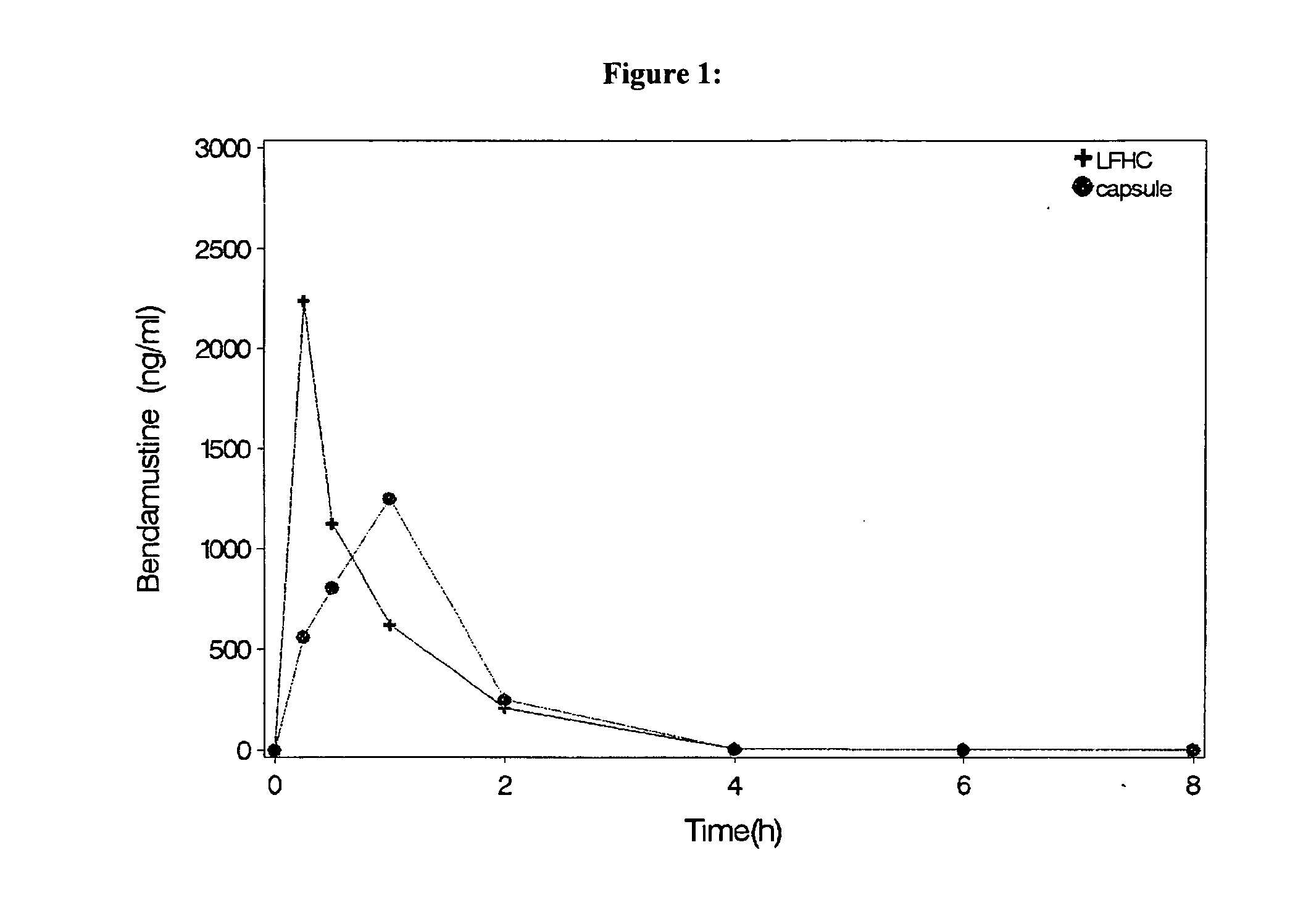
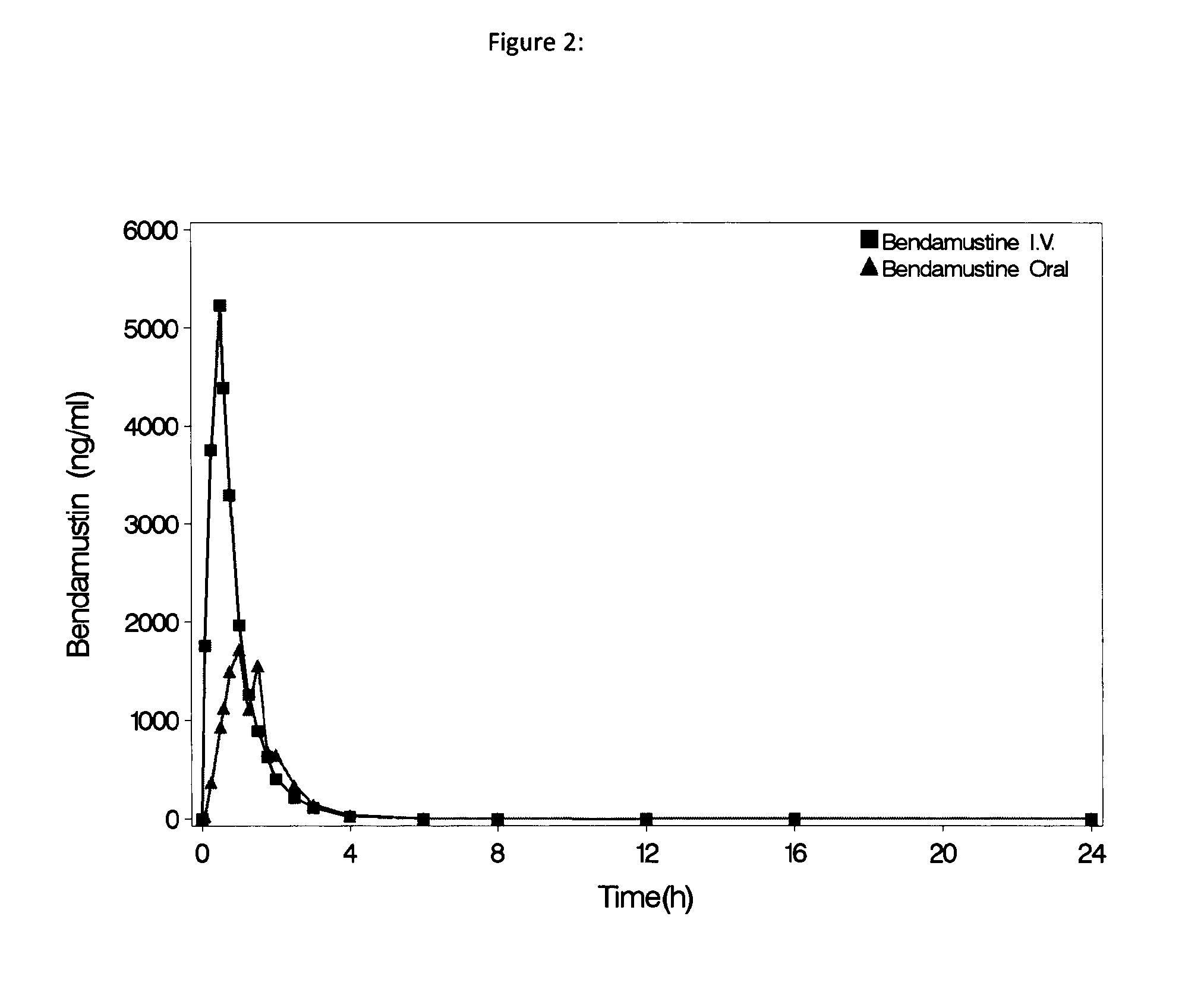
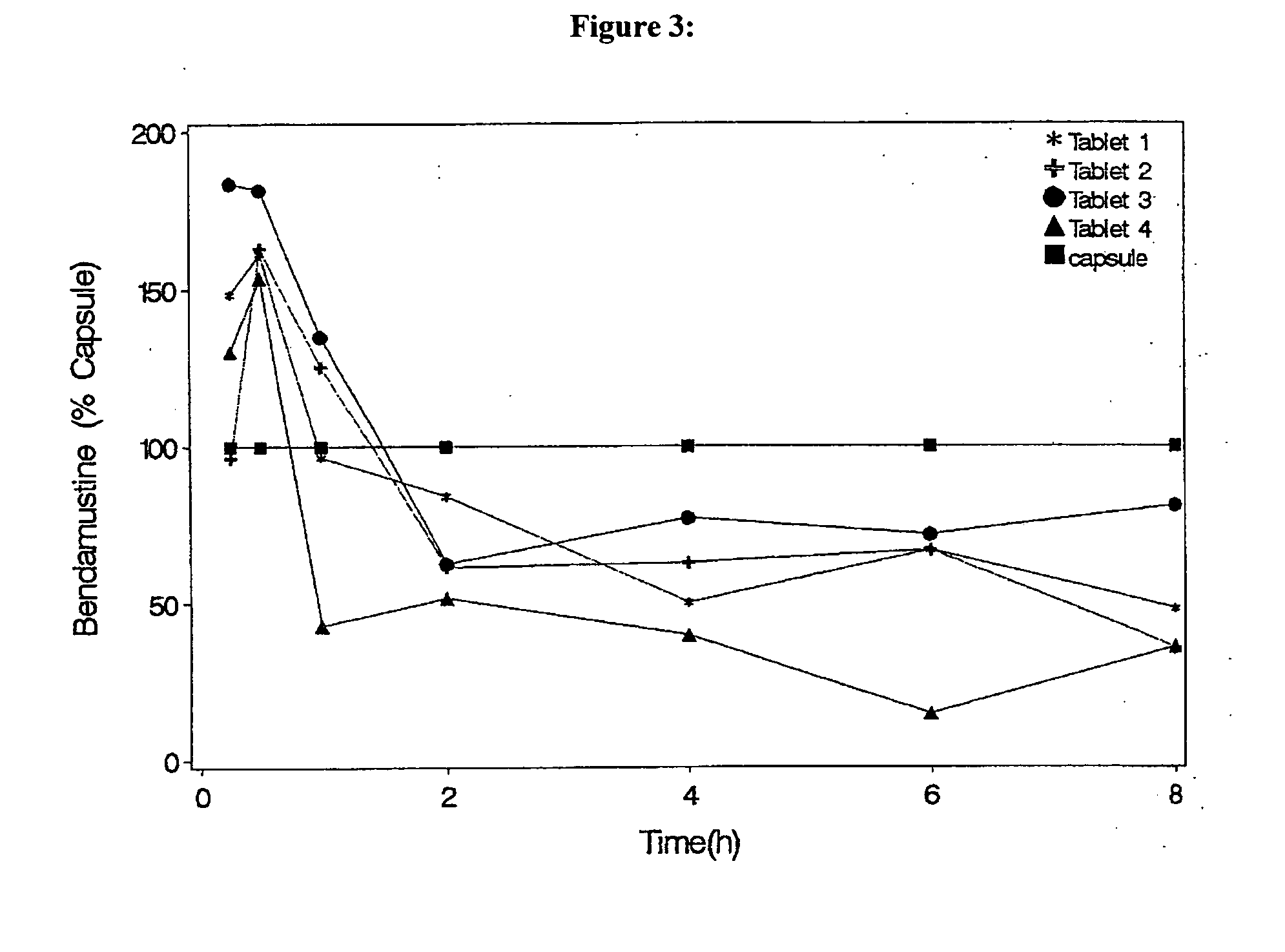
Oral Dosage Forms of Bendamustine and Therapeutic Use Thereof
InactiveUS20130209558A1Improve stabilitySuperior dissolution profileBiocideHeavy metal active ingredientsDiseaseOral treatment
In the present invention there is provided a pharmaceutical composition for oral administration which comprises bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient and which shows a dissolution of the bendamustine of at least 60% in 20 minutes, 70% in 40 minutes and 80% in 60 minutes, as measured with a paddle apparatus at 50 rpm according to the European Pharmacopoeia in 500 ml of a dissolution medium at a pH of 1.5, and wherein the pharmaceutically acceptable excipient is either a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of a polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide or a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5. The invention further relates to the above pharmaceutical composition for use for the oral treatment of a medical condition which is selected from chronic lymphocytic leukemia, acute lymphocytic leukaemia, chronic myelocytic leukaemia, acute myelocytic leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, breast cancer, ovarian cancer, small cell lung cancer and non-small cell lung cancer. The invention moreover relates to the above pharmaceutical composition for the above use wherein the dosage regimen comprises at least the administration of a dose of 100 to 600 mg / m2 / per person of bendamustine on day 1 and day 2, optionally a dose of 50 to 150 mg / m2 i.v. or orally of a corticosteroid on days 1 to 5, and optionally a suitable dose of a further active agent selected from the group consisting of an antibody specific for CD20, an anthracyclin derivative, a vinca alkaloid or a platin derivative; and the repetition of said dosage regimen 4 to 15 times after intervals of two to four weeks.
Owner:ASTELLAS DEUTLAND
Nano-Emulsion Injection of Vinca Alkaloids and the Preparation Method Thereof
InactiveUS20120045489A1Low toxicityHigh encapsulation efficiencyBiocideNanomedicineOil phaseHigh pressure
A nano-emulsion injection of Vinca alkaloids and its preparation method are disclosed. The injection is an oil-in-water emulsion injection comprising Vinca alkaloids or their salts, injectable oil, surfactant(s) and injectable water, wherein the average diameter of the droplets of the emulsion is less than 100 nm and the pH of the emulsion is 7-9. The preparation method comprises the steps of preparing the oil phase and the aqueous phase respectively, homogeneously mixing the oil phase and the aqueous phase with high speed, adding the active ingredient, adjusting the pH to 7-9, adding water to constant volume, and homogenizing the emulsion till the average diameter of the droplets being less than 100 nm. The alternative method comprises the steps of homogeneously mixing the oil phase and the aqueous phase, homogenizing the obtained emulsion under high pressure till the average diameter of the droplets being less than 100 nm, adding the active ingredient, adjusting the pH to 7-9, stirring, and adding water to constant volume.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Pharmaceutical composition
InactiveUS20100160208A1Solution to short lifeReduce dosageAntibacterial agentsSenses disorderMessenger RNAPurine
Owner:ANTISENSE PHARMA GMBH
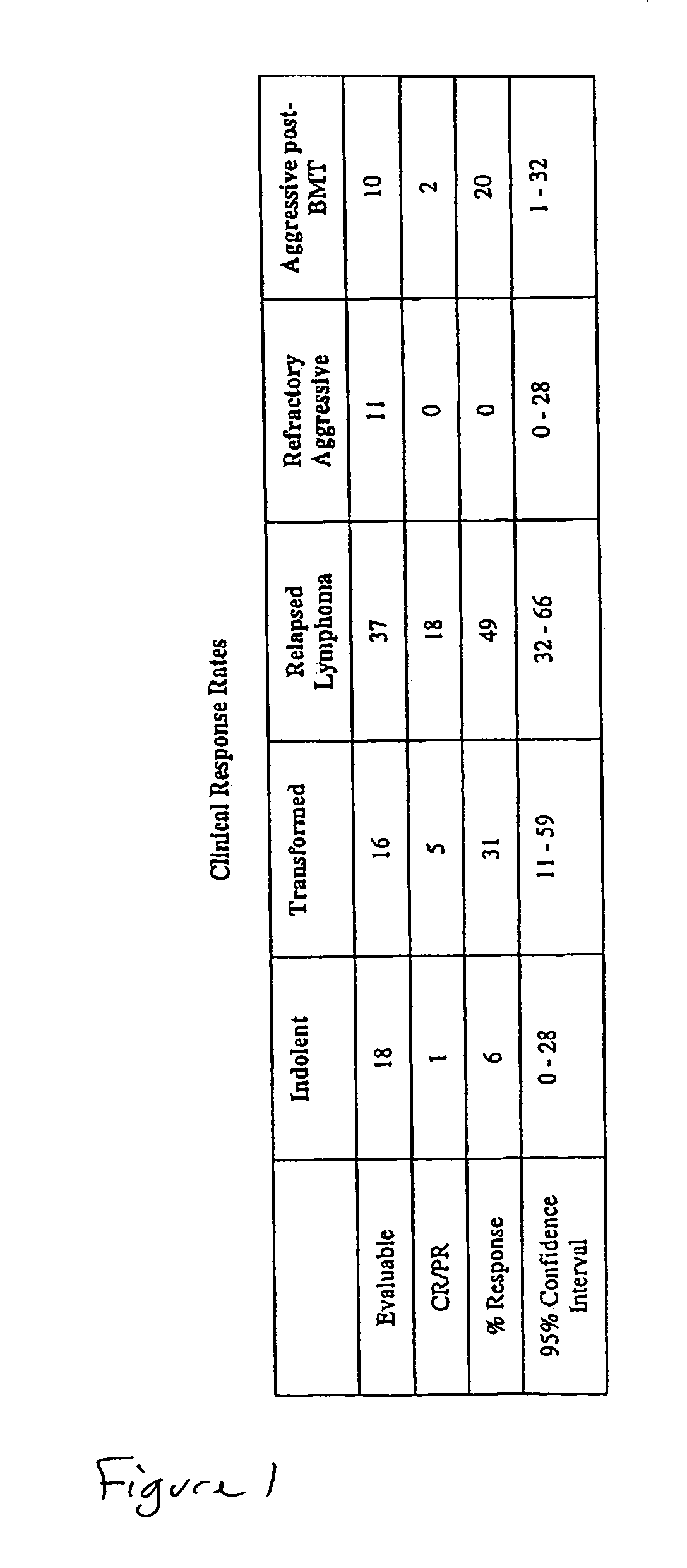
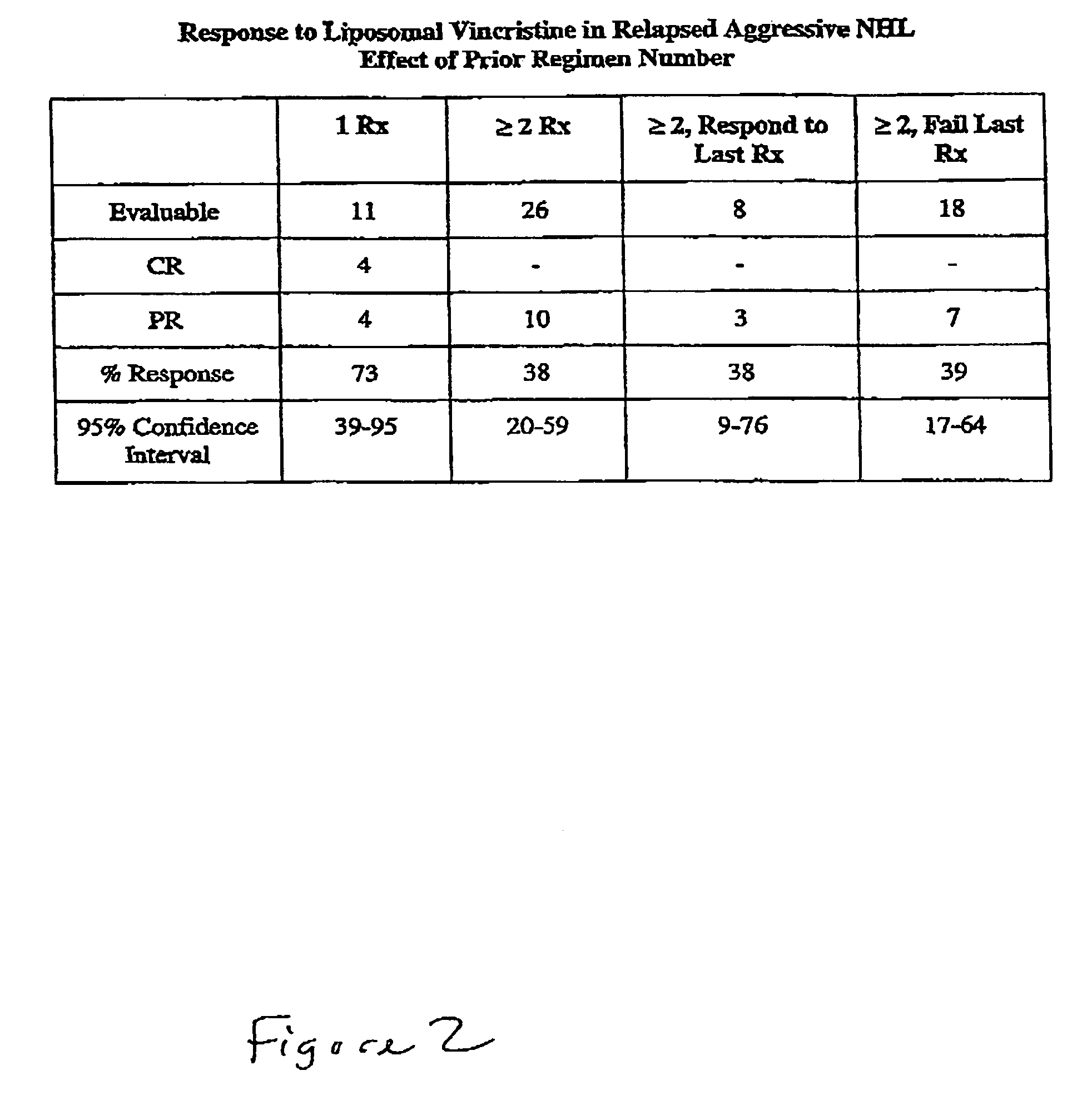
Compositions and methods for treating lymphoma
InactiveUS7244450B2Increase cancerous propertyOrganic active ingredientsLiposomal deliveryMedicineLiposome
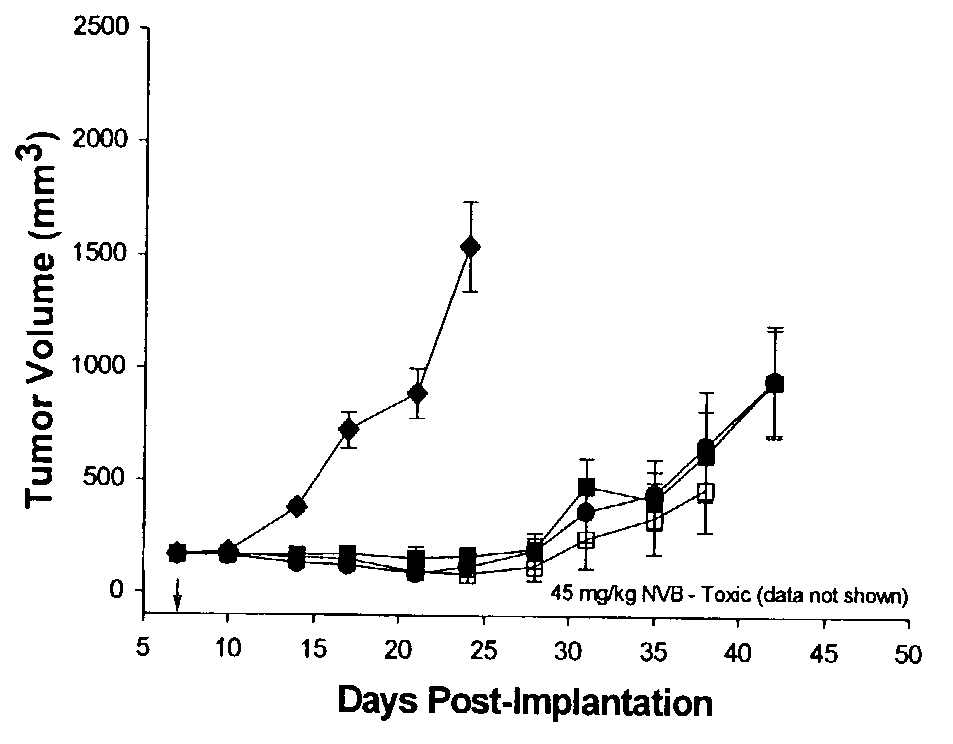
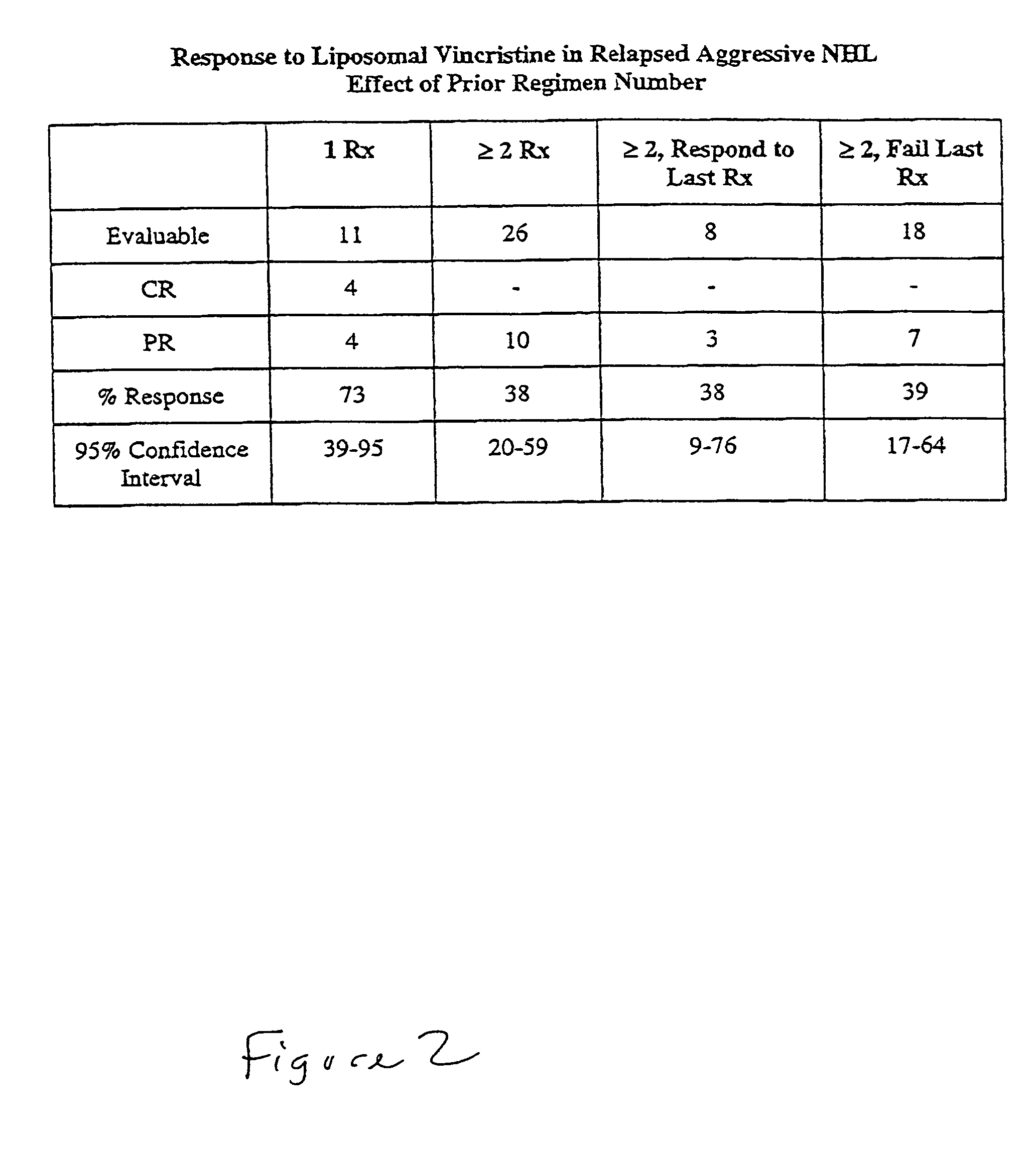
This invention provides methods for treating neoplasias in a mammal. In particular, the invention provides methods for treating various types of lymphomas, including relapsed forms of non-Hodgkin's Lymphoma. These methods involve the administration of liposome-encapsulated vinca alkaloids, e.g., vincristine, to a mammal with a lymphoma.
Owner:ACROTECH BIOPHARMA LLC +2
Vinca derivatives
Owner:ALBANY MOLECULAR RESEARCH INC
Separation and purification method for vinca alkaloids
ActiveCN101638413AHigh degree of automationEconomic industrial productionOrganic chemistryChromatographic separationPurification methods
A separation and purification method for vinca alkaloids comprises the following steps: (1) the pretreatment of coarse crude products: putting vinca alkaloids crude product on an aluminum oxide column, eluting and collecting alkaloid fraction, combining and then decompressing and concentrating till being dry to obtain a pretreated product; (2) high performance liquid chromatography separation: firstly preparing chromatographic column stationary phase, filling being silica gel, sample handling, gradient elution, following the situation of the preparative chromatography separation by a detector,thin layer chromatography or high efficient liquid phase detection, and combining enriched vinca alkaloids fraction; (3) recrystallization: decompressing and concentrating alkaloid fraction till being dry, dissolving lower alkyl alcohols, laying aside, crystallizing, filtering and drying. The method is suitable for the separation and purification of the vinca alkaloids, does not need particular filling, also does not need to use high toxicity solvent, is simple to operate, has high automatic degree, good separation effect; the filling used by stationary phase is packed into the column at onetime and used for many times. Mobile phase can be recycled, is environmental friendly, economic and suitable for industrialization production.
Owner:GUANGZHOU HANFANG PHARMA
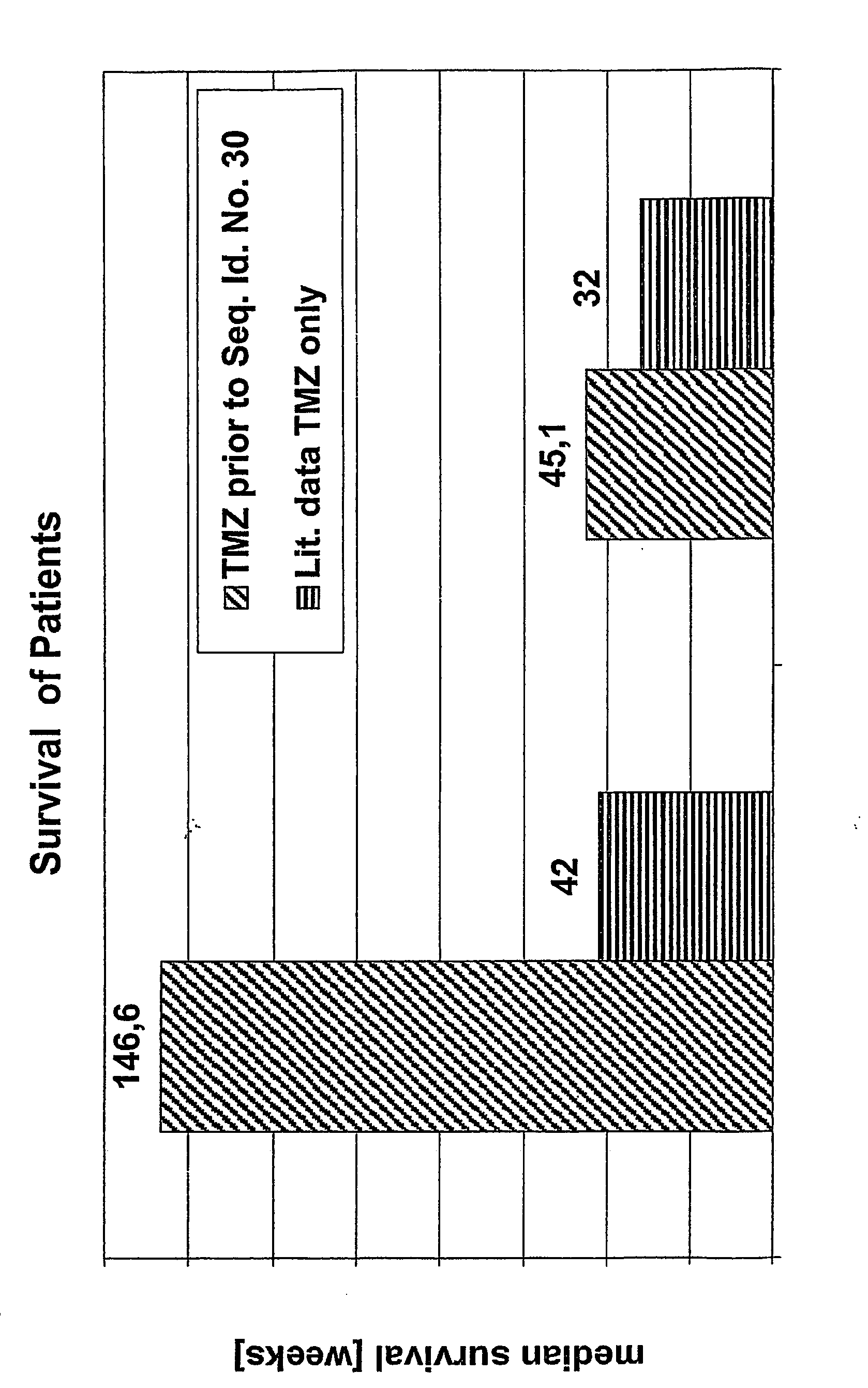
Pharmaceutical composition
InactiveUS20070196269A1Solution to short lifeReduce dosageAntibacterial agentsOrganic active ingredientsMessenger RNAPurine
The invention concerns a pharmaceutical composition comprising at least one stimulator of the immune cell functions and at least one substance inhibiting the cell proliferation and / or inducing cell death. In a preferred embodiment the stimulator of the function of the immune system and / or the immune cells are antagonists of TGF-beta selected from the group of oligonucleotides hybridizing with an area of the messenger RNA and or DNA encoding TGF-beta and the at least one substance inhibiting cell proliferation and / or inducing cell death is selected from the group of temozolomide, nitrosoureas, Vinca alkaloids, antagonists of the purine and pyrimidines bases, cytoststatic active antibiotics, caphthotecine derivatives, anti estrogens, anti-androgens and analogs of gonadotropin releasing hormon.
Owner:ANTISENSE PHARMA GMBH
Vinca derivatives
InactiveUS20050137169A1Inhibit cell proliferationAntibacterial agentsBiocideMedicinal chemistryVinca alkaloid
Owner:ALBANY MOLECULAR RESEARCH INC
Sigma ligands for the prevention or treatment of pain induced by chemotherapy
InactiveUS20120141606A1Avoid developmentEffective treatmentBiocideHeavy metal active ingredientsPlatinumMedicine
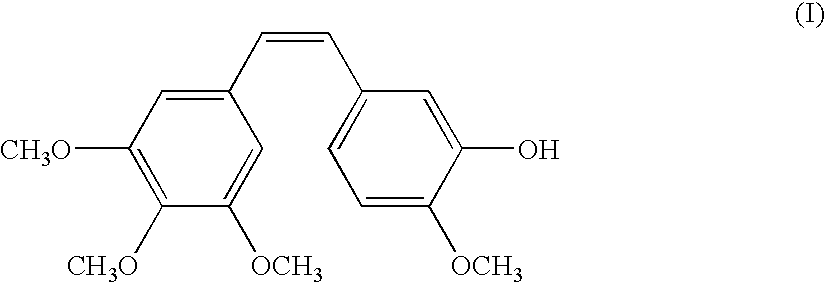
The invention refers to the use of a sigma ligand of formula (I) to prevent or treat pain induced by a chemotherapeutic agent, especially pain induced by taxanes, vinca alkaloids or platinum-containing chemotherapeutic drugs.
Owner:LAB DEL DR ESTEVE SA
Compositions and methods for treating cancer
This invention provides compositions and methods for treating neoplasias in a mammal. In particular, the invention provides liposome-encapsulated vinca alkaloids, e.g., vinorelbine, and methods of treating a mammal using such compositions.
Owner:UNIV OF TEXAS MD ANDERSON CANCER CENT THE +1
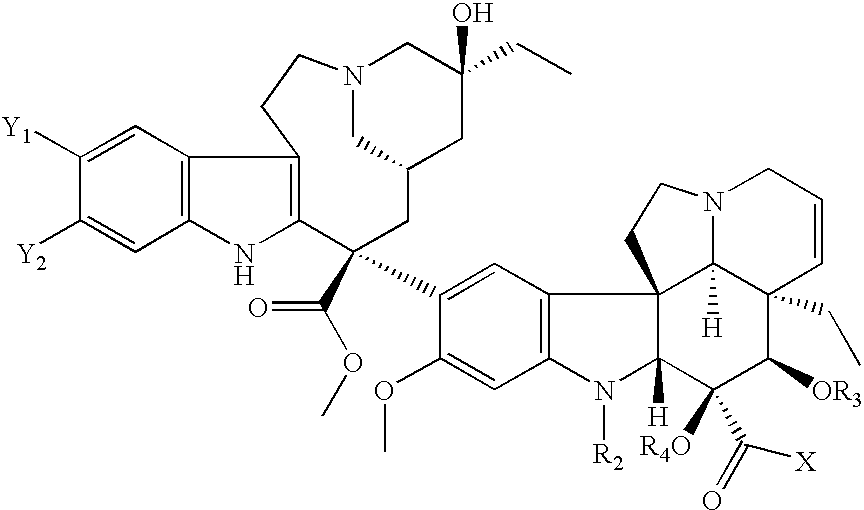
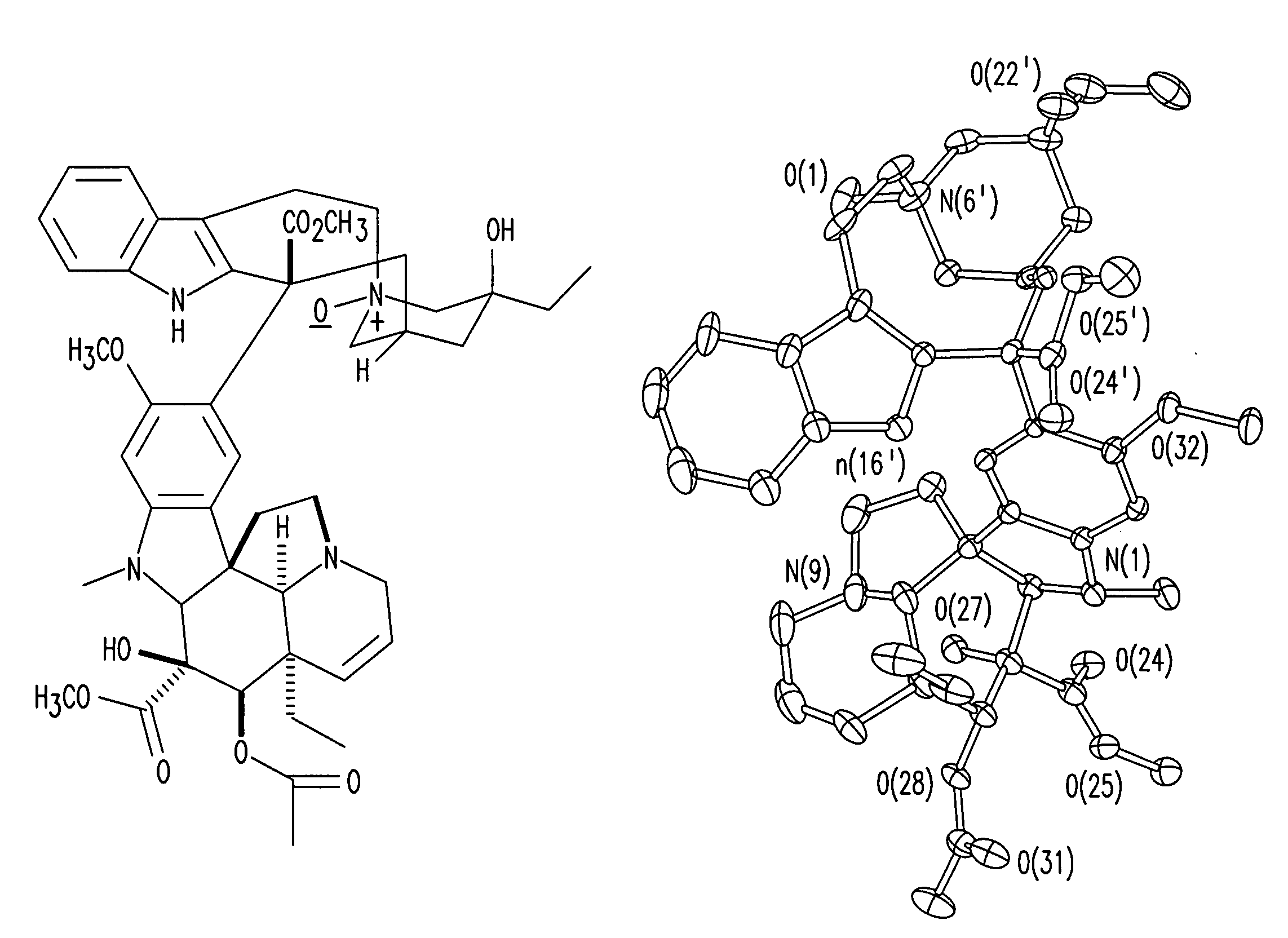
Treatment of Hyperproliferative Diseases with Vinca Alkaloid N-Oxide and Analogs
ActiveUS20080305075A1Prevent and ameliorate side effectReduce riskBiocideSenses disorderDiseaseActive agent
The present invention relates to vinca alkaloid and analog N-oxides having activity for treating hyperproliferative disorders. Further, the invention relates to pharmaceutical compositions and methods of using vinca alkaloid and analog N-oxides, alone or in combination with one or more other active agents or treatments, to treat hyperproliferative disorders.
Owner:CASCADE PRODRUG
Method for preventing and/or treating peripheral neuropathies induced by the administration of an anticancer agent
InactiveUS20030199535A1Eliminate the effects ofEliminate side effectsBiocideHeavy metal active ingredientsAnticarcinogenSide effect
A method for preventing and / or treating peripheral neuropathies induced by the administration of an anticancer agent of the family of platin compounds, taxanes, epothilone class, vinca alkaloids, said method comprising the administration in a co-ordinated manner to a subject suffering from said peripheral neuropathies, or expected to suffer from said peripheral neuropathies, an effective amount of acetyl L-carnitine or of a pharmaceutically acceptable salt thereof. In case of prevention, acetyl L-carnitine is administered to a subject, in view of the need of a treatment with an anticancer agent, immediately before or immediately after surgical removal of the tumor, but in any case before the administration of the anticancer agent. Acetyl L-carnitine can enhance the supportive effect of physiological NGF during chemotherapy-induced neuropathy, thus avoiding the problem of the local and general side effects of the exogenous administration of NGF which are a major problem of this neuroprotective strategy.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Method for extracting vinca alkaloids extract from Vinca rosea
InactiveCN101157699AIncrease profitEmission reductionOrganic chemistryPlant ingredientsCatharanthineFiltration
The invention relates to a method to extract the vinblastine extracted from the catharanthus roseus, including the following processes: (1) the catharanthus roseus is agitated and smashed after the wetting by the acidic water and then is soaked in the acidic water, the filtrate after the filtration is regulated to alkalinity by using alkali, a alkaline water phase is extracted by using chloroform, a chloroform phase is collected, and the solid reclaimed materials can be obtained after the reclaiming of the chloroform; (2) the reclaimed materials are dissolved by using the acidic water, the ethyl acetate is added for extraction, and a acidic water phase is reclaimed; (3) the reclaimed acidic water phase in step (2) is regulated to alkalinity, the chloroform is used for extraction, and the crude alkaloid is obtained by the reclaim of chloroform of the extraction phase; (4) the obtained crude alkaloid in step (3) is dissolved by using the acidic water, of which the pH is regulated to alkalinity, the lower layer of sediments is collected centrifugally, the refined catharanthus roseus alkaloid is obtained after the drying of the sediments, and the vinblastine and leurocristine can be obtained by alumina column chromatography separation; (5) the filtrate which is obtained by centrifugation in step (4) is extracted by using chloroform, the catharanthine and the vindoline are obtained by vacuum concentration and macroporous resin separation. The method can improve the utilization ratio of catharanthus roseus.
Owner:刘全胜
Use of Vinca Alkaloids and Salts Thereof
InactiveUS20070232533A1Increase insulin productionIncreasing insulin-producingBiocideSenses disorderMedicinePancreas
An agent containing a vinca alkaloid or its pharmacologically acceptable salt as an active ingredient can induce insulin production and / or secretion of non-neoplastic cells derived from the pancreas.
Owner:KEIO UNIV
Compositions and methods for treating lymphoma
InactiveUS20090028933A1Increase cancerous propertyOrganic active ingredientsBiocideDexamethasoneOncology
The present invention provides methods for treating neoplasias in a mammal. In particular, the invention provides methods for treating various types of leukemias, including acute lymphoblastic leukemia (ALL). These methods involve the administration of liposome-encapsulated vinca alkaloids, e.g., vincristine, in combination with dexamethasone to a mammal with a leukemia.
Owner:TALON THERAPEUTICS
Antiproliferative combination comprising cyc-682 and a cytotoxic agent
A first aspect of the invention relates to a combination comprising 2′-cyano-2′-deoxy-N4-palmitoyl-1-beta-D-arabi-nofuranosyl-cytosine, or a metabolite thereof, or a pharmaceutically acceptable salt thereof, and a cytotoxic agent selected from (a) a vinca alkaloid; (b) a taxane; (c) a cytosine analogue; (d) an anthracycline; and (e) a platinum antineoplastic agent. A second aspect of the invention relates to a pharmaceutical product comprising the above combination as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method for treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering the above combination.
Owner:CYCLACEL
Enhanced b cell cytotoxicity of cdim binding antibody
InactiveUS20100322849A1Organic active ingredientsIn-vivo radioactive preparationsDiseaseAutoimmune disease
Formulations and methods of treating human patients suffering from a condition characterized by lymphoid cancer, autoimmune disease or B cell hyperproliferation are disclosed, the treatment comprising administering (1) a cytotoxic amount of an antibody having specific binding for CDIM epitopes on a B cell, and (2) a cytotoxic agent, including a chemotherapeutic agent, radioactive isotope, cytotoxic antibody, immunoconjugate, ligand conjugate, immunosuppressant, cell growth regulator and / or inhibitor, toxin, or mixtures thereof, including agents that disrupt the cytoskeleton of B cells, particularly vinca alkaloids or colchicine.
Owner:BHAT NEELIMA M +3
Combination comprising combretastatin and anticancer agents
An antitumor combination comprising a stilbene derivative and an anticancer compound selected from the group consisting of taxanes, alkylating agents, antimetabolites, vinca alkaloids, platinum compounds, epidophylloptoxins, and antibiotics as the active ingredients is provided. Methods of using these pharmaceutical preparations for the treatment of solid carcinomas and the like are also provided.
Owner:AVENTIS PHARMA SA
Method for preventing and/or treating peripheral neuropathies induced by the administration of an anticancer agent
InactiveUS20060183798A1Quality improvementProlong lifeBiocideHeavy metal active ingredientsAbnormal tissue growthDisease
A method for preventing and / or treating peripheral neuropathies induced by the administration of an anticancer agent of the family of platin compounds, taxanes, epothilone class, vinca alkaloids, said method comprising the administration in a co-ordinated manner to a subject suffering from said peripheral neuropathies, or expected to suffer from said peripheral neuropathies, an effective amount of acetyl L-carnitine or of a pharmaceutically acceptable salt thereof. In case of prevention, acetyl L-carnitine is administered to a subject, in view of the need of a treatment with an anticancer agent, immediately before or immediately after surgical removal of the tumor, but in any case before the administration of the anticancer agent. Acetyl L-carnitine can enhance the supportive effect of physiological NGF during chemotherapy-induced neuropathy, thus avoiding the problem of the local and general side effects of the exogenous administration of NGF which are a major problem of this neuroprotective strategy.
Owner:CAVAZZA CLAUDIO +2
Enhancing treatment of MDR cancer with adenosine A3 antagonists
The present invention discloses the use of high affinity adenosine A3 receptor antagonists for enhancing chemotherapeutic treatment of cancers expressing adenosine A3 receptors and cancers expressing P-glycoprotein or MRP. In preferred embodiments, adenosine A3 receptor antagonists are administered before or during administration of a taxane family, vinca alkaloid, camptothecin or antibiotic chemotherapeutic agent.
Owner:KING PHARMA RES & DEV
Compositions and methods for treating lymphoma
InactiveUS7247316B2Increase cancerous propertyImprove abilitiesBiocideAnimal repellantsMedicineLiposome
This invention provides methods for treating neoplasias in a mammal. In particular, the invention provides methods for treating various types of lymphomas, including relapsed forms of non-Hodgkin's Lymphoma. These methods involve the administration of liposome-encapsulated vinca alkaloids, e.g., vincristine, to a mammal with a lymphoma.
Owner:TALON THERAPEUTICS +2
Vinca alkaloid nano emulsion injection and preparation method thereof
InactiveCN101879138ALess irritatingReduce viscosityOrganic active ingredientsEmulsion deliveryVinca alkaloidNanotechnology
The invention provides a nano emulsion injection containing vinca alkaloid, especially vinorelbine, and a preparation method thereof. The nano emulsion injection has the average grain diameter of smaller than 100nm, not only can reduce the pharmaceutical simulation of a vinorelbine injection but also can improve the concentration of the vinorelbine in a product and meanwhile has higher targeted performance.
Owner:SHANGHAI HENGRUI PHARM CO LTD
Compositions for delivering highly water soluble drugs
Owner:MAST THERAPEUTICS
Compositions and methods for treating lymphoma
Owner:ACROTECH BIOPHARMA LLC +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com