Method for synthesizing 1 beta methyl carbapenem antibiotic
A technology of methyl carbapenem and synthesis method, applied in the directions of bulk chemical production, organic chemistry, etc., can solve problems such as low efficiency, unfavorable large-scale production, etc., and achieve the effect of simplifying the operation process and being beneficial to large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
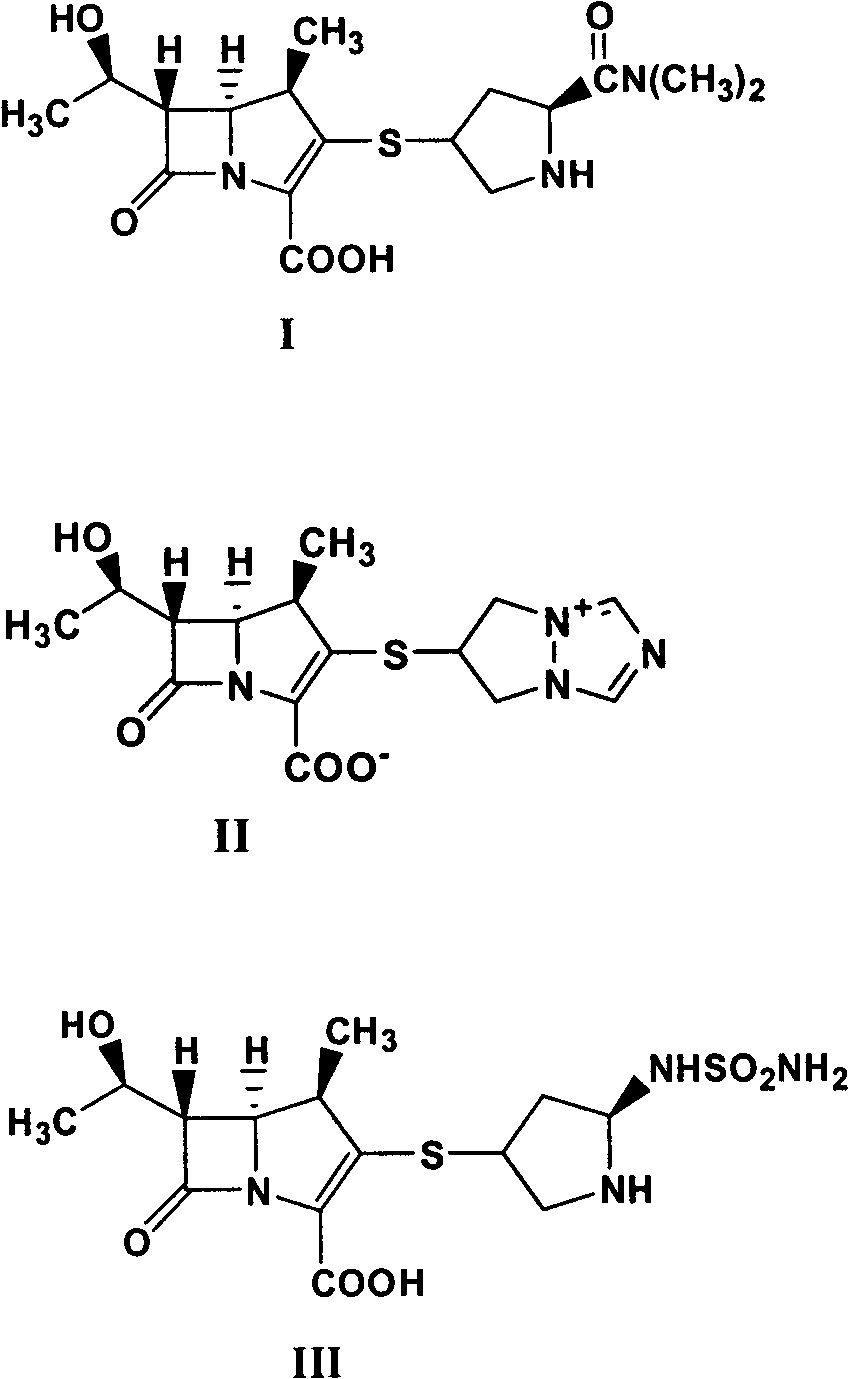
[0062] Preparation of Meropenem (Compound I)
[0063]
[0064]10.0 g of meropenem (compound Ia), 300.0 g of acetonitrile, 300 g of water, 5.5 g of 3,5,-lutidine, and 4.8 g of 5% palladium carbon were added to a 2L autoclave, and the air was removed. Introduce hydrogen, react at room temperature for 6 hours, after the reaction is complete, collect palladium carbon by filtration, add 400 g of acetone to the filtrate, stir and crystallize at -15°C for 20 hours, filter, and dry the product to obtain 5.7 g of meropenem (compound I) trihydrate , The mass yield is 57.0%.
[0065] The target compound (compound I) is a known substance, and the retention time and mass spectrum of the target product obtained in Example 1 in HPLC are consistent with the existing literature.
Embodiment 2
[0067] Preparation of Meropenem (Compound I)
[0068]
[0069] Add 12.0 g of meropenem (compound Ia), 300.0 g of acetone, 300 g of water, 5.5 g of 3,5,-lutidine, and 4.8 g of 5% palladium carbon into a 2L autoclave, remove the air, Introduce hydrogen and react at room temperature for 10 hours. After the reaction is complete, collect palladium carbon by filtration, add 400 g of acetone to the filtrate, stir and crystallize at -20°C for 20 hours, filter, and dry the product to obtain 5.9 g of meropenem (compound I) trihydrate , The mass yield is 49.2%.
[0070] The target compound (compound I) is a known substance, and the retention time and mass spectrum of the target product obtained in Example 2 in HPLC are consistent with the existing literature.
Embodiment 3
[0072] Preparation of Meropenem (Compound I)
[0073]
[0074] Put 20.0 g of meropenem (compound Ia), 600.0 g of acetonitrile, 600 g of water, 5.5 g of 3,5,-lutidine, and 8.5 g of palladium carbon into a 2L autoclave, remove the air, and feed Hydrogen, react at room temperature for 6 hours, after the reaction is complete, collect palladium carbon by filtration, add 400g of acetonitrile to the filtrate, stir and crystallize at 0°C for 24 hours, filter, and dry the product to obtain 9.8g of meropenem (Compound I) trihydrate, the mass yield Rate 49.0%.
[0075] The target compound (compound I) is a known substance, and the retention time and mass spectrum of the target product obtained in Example 3 in HPLC are consistent with the existing literature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com