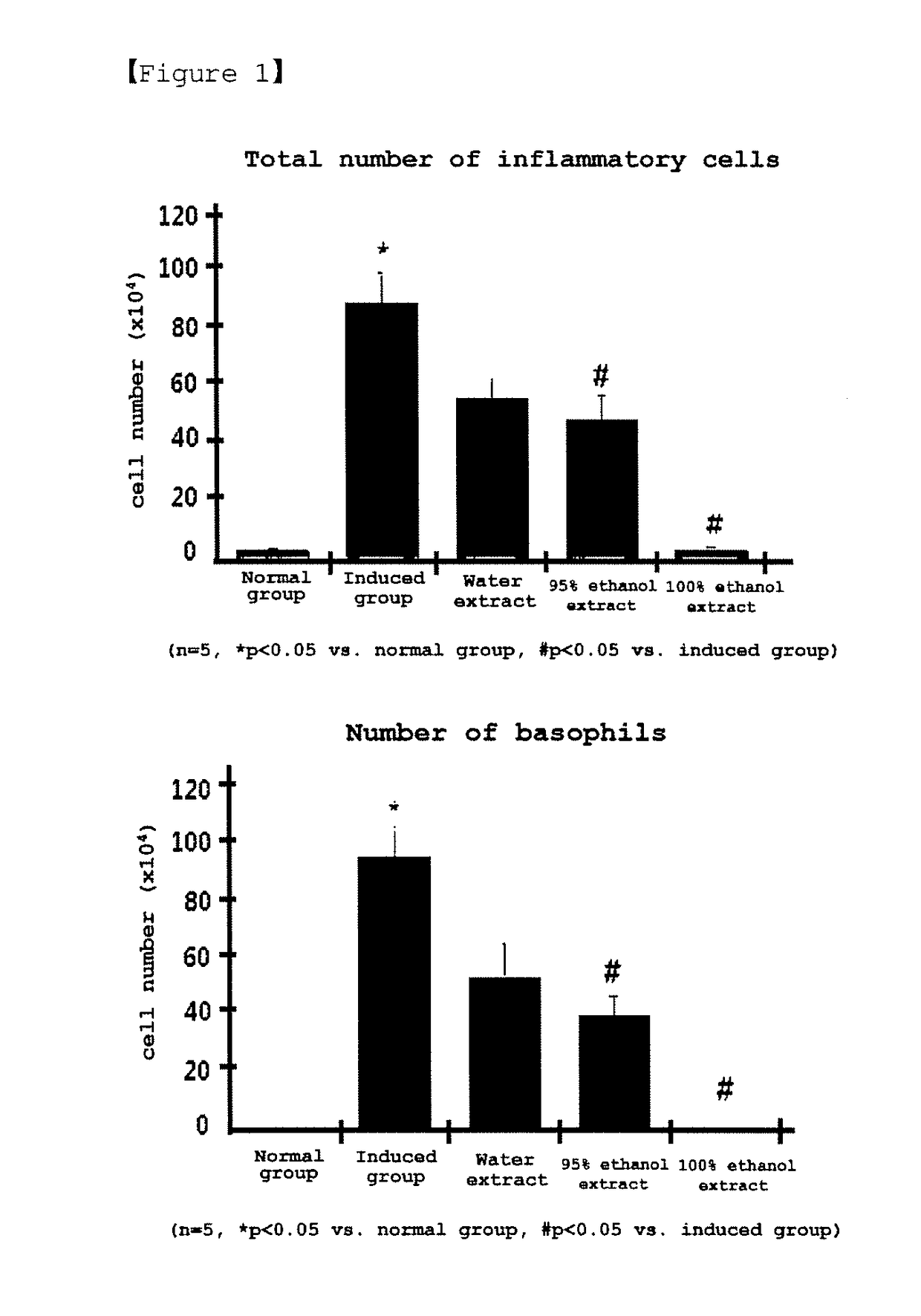
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "Basophil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Basophils are a type of white blood cell. Basophils are the least common of the granulocytes, representing about 0.5% to 1% of circulating white blood cells. However, they are the largest type of granulocyte. They are responsible for inflammatory reactions during immune response, as well as in the formation of acute and chronic allergic diseases, including anaphylaxis, asthma, atopic dermatitis and hay fever. They also produce compounds that co-ordinate immune responses, including histamine and serotonin that induce inflammation, heparin that prevents blood clotting, although there are less than that found in mast cell granules. It used to be thought that basophils that have migrated from blood into their resident tissues (connective tissue) are known as mast cells, but this is no longer thought to be the case.
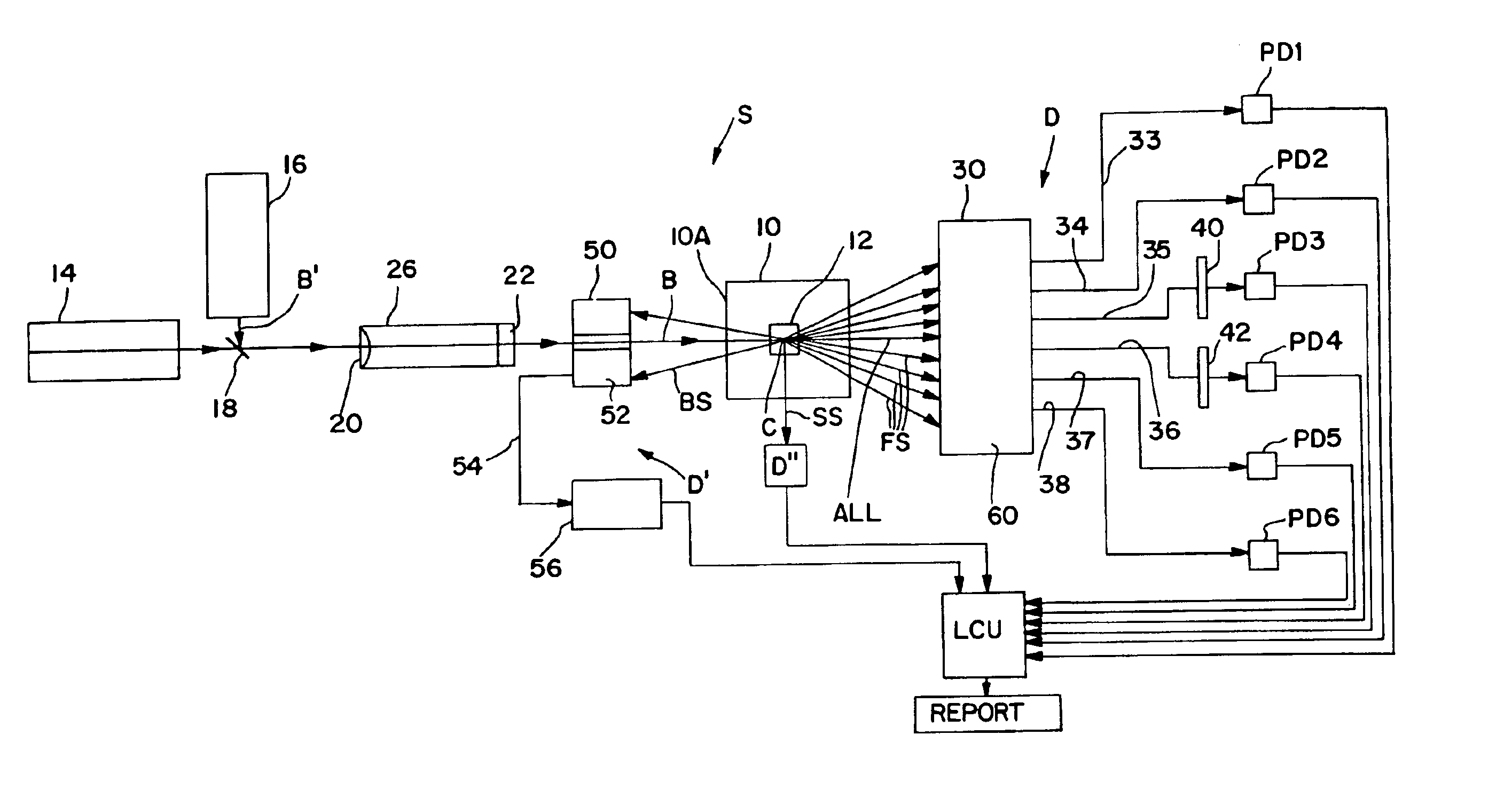
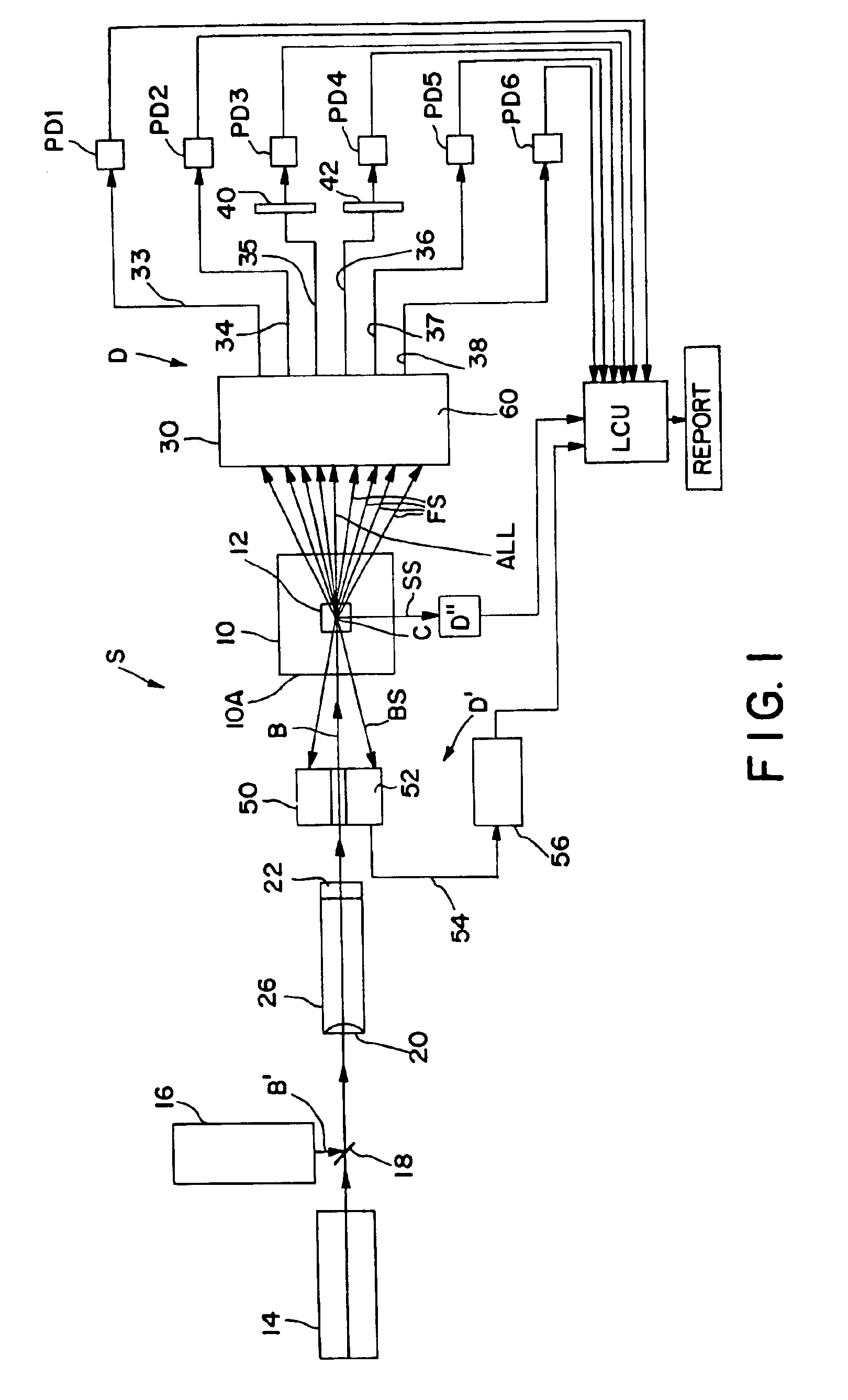
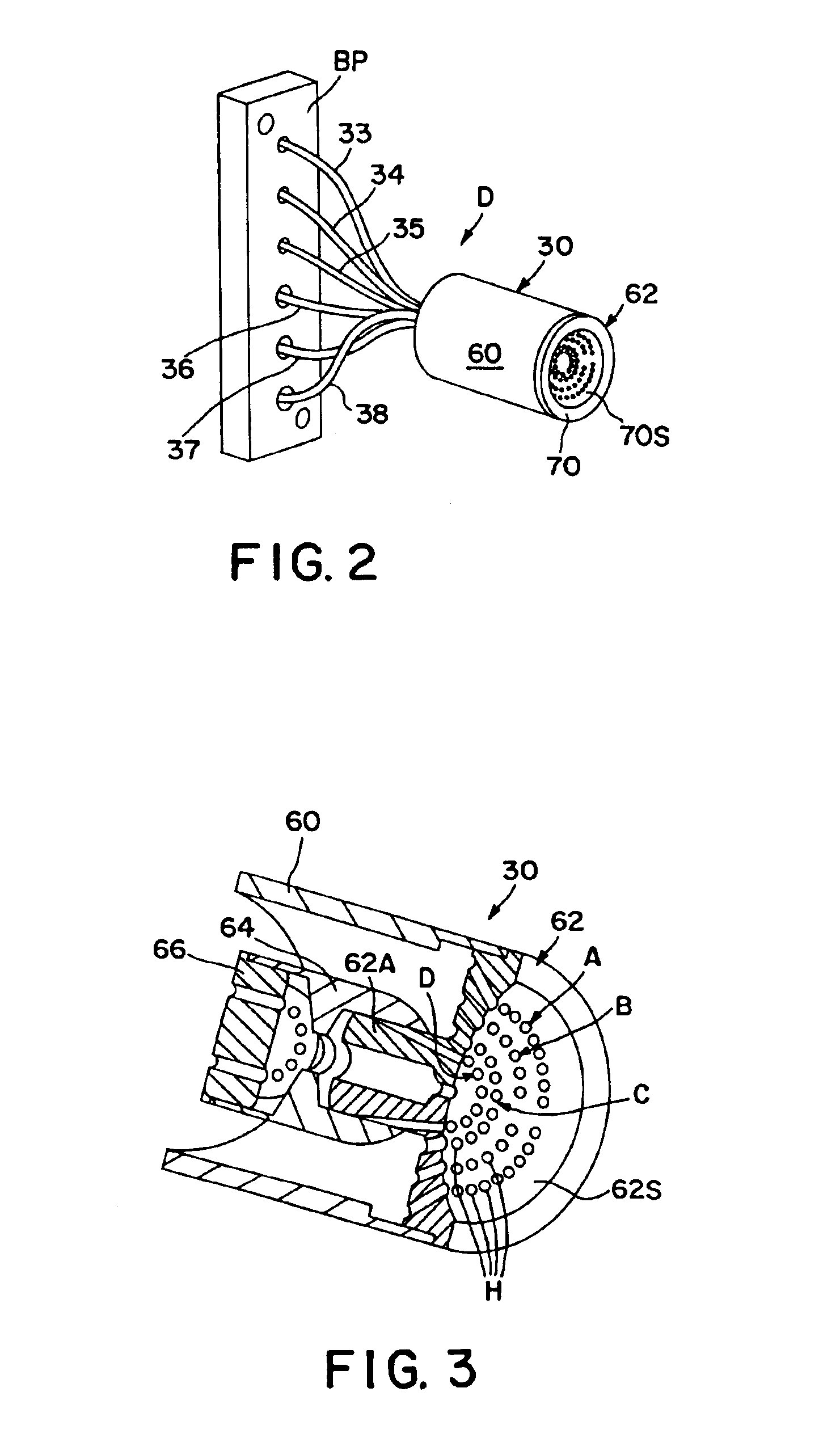
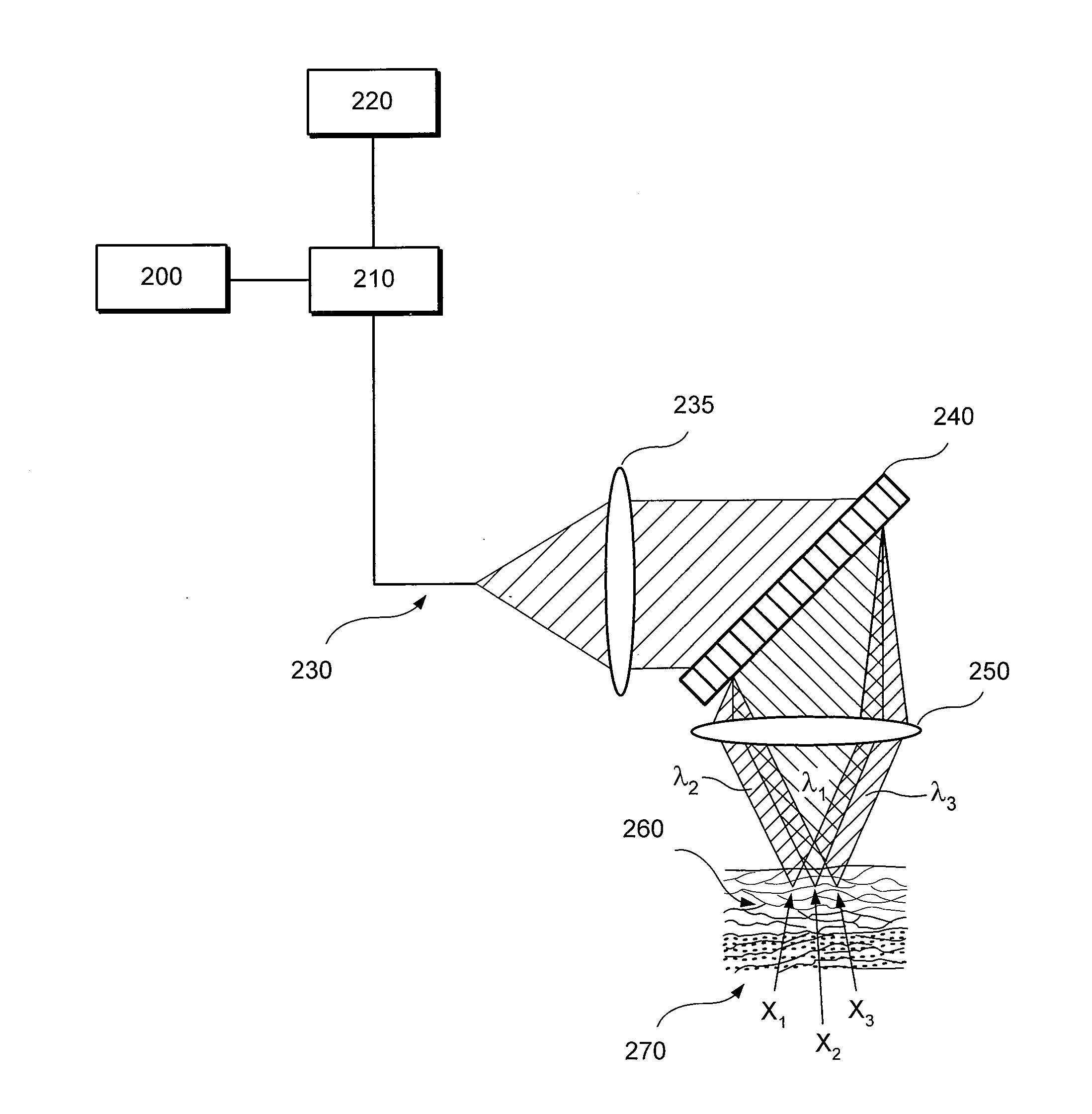
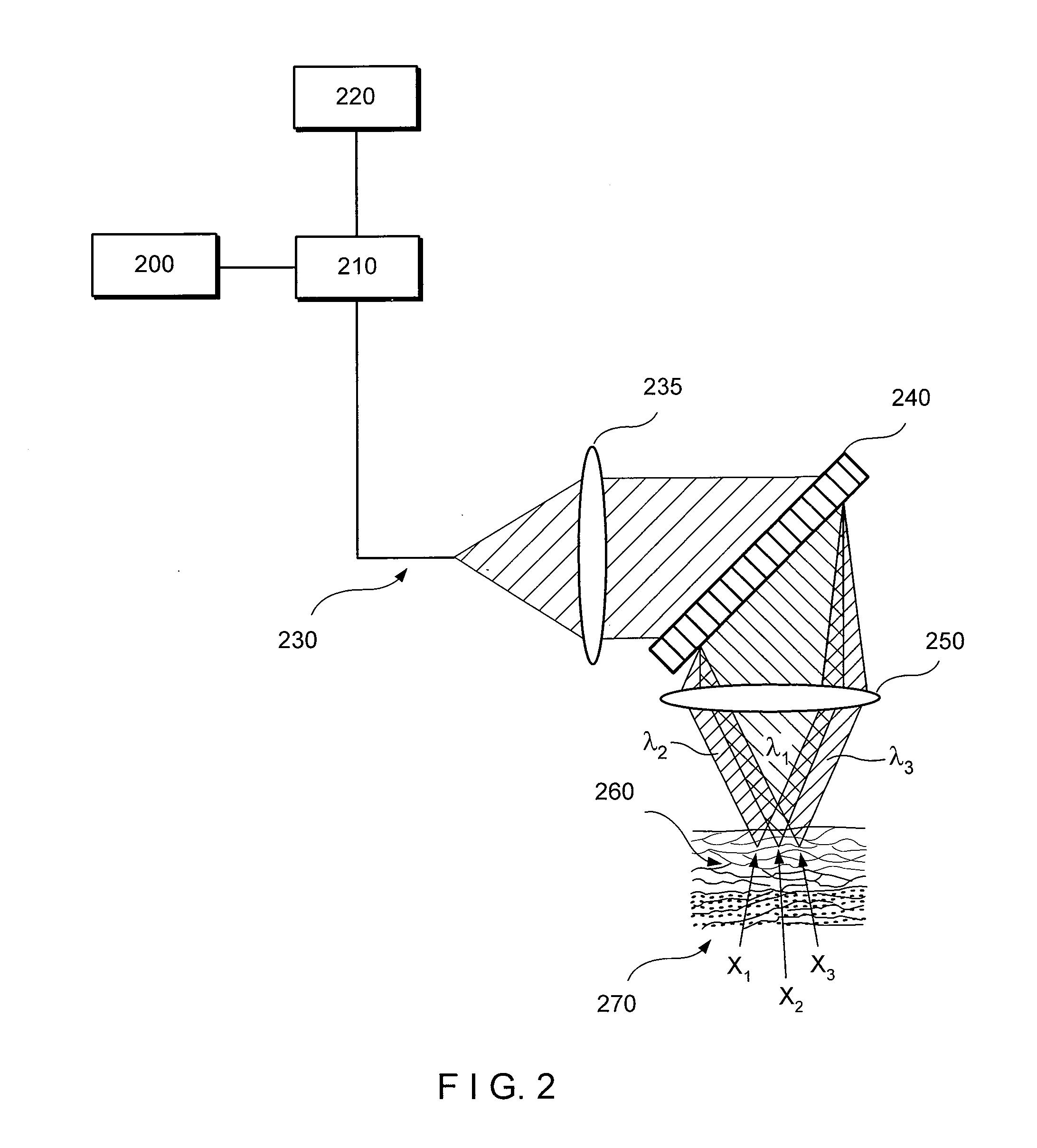
Apparatus for differentiating blood cells using back-scatter
InactiveUS6869569B2Material thermal conductivityMaterial analysis by electric/magnetic meansPhotodetectorRed blood cell
Blood cells of interest are readily distinguishable from other blood cells and look-a-like particles found in a blood sample by their back-scatter signature. A preferred method for differentiating platelets in a blood sample is to irradiate the cells and particles, one at a time, with a beam of radiation, and to detect back-scattered (reflected) radiation using a plurality of optical fibers to transmit the back-scattered radiation to a high-gain photodetector, e.g. a photomultiplier tube. Preferably, the back-scatter signal so obtained is combined with a second signal representing, for example, either the level of forward-scatter within a prescribed, relatively narrow angular range, or the level of side-scattered radiation, or the level of attenuation of the cell-irradiating beam caused by the presence of the irradiated cell or particle in the beam, or the electrical impedance of the irradiated cell or particle, to differentiate the cells of interest. The method and apparatus of the invention are particularly useful in differentiating platelets and basophils in a blood sample.
Owner:COULTER INTERNATIONAL CORPORATION
Methods and assays for detecting and quantifying pure subpopulations of white blood cells in immune system disorders
ActiveUS20100112628A1Determining susceptibility to an allergic reactionBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseWhite blood cell
Methods for detecting nonactivated basophils in a whole blood sample obtained from a normal healthy subject, methods for determining a subject's susceptibility to an allergic reaction to an allergen, where the subject has no known allergy to the allergen, methods for measuring a response to challenge with a potential allergen in a whole blood sample obtained from a subject with known allergic reactivity to allergens other than the potential allergen; and an in vitro system for reliable detection or quantification of a specific white blood cell population in a whole blood sample are described.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Devices and methods for imaging particular cells including eosinophils
InactiveUS20110137178A1Easy to installFaster and high resolutionDiagnostics using spectroscopyDianostics using fluorescence emissionMast cellEosinophil
An exemplary embodiment of apparatus and method according to the present disclosure can be provided. For example, using at least one first arrangement, it is possible to direct at least one first electro-magnetic radiation to at least one portion of tissue within a body. Using at least one second arrangement, it is possible to receive at least one second electro-magnetic radiation provided from the portion, which is based on the first electro-magnetic radiation. Further, using at least one third arrangement, it is possible to differentiate at least one particular cell which is eosinophil, mast cell, basophil, monocyte and / or nutrophil from other cells in the portion based on the second electro-magnetic radiation.
Owner:THE GENERAL HOSPITAL CORP
Production of cultured human mast cells and basophils for high throughput small molecule drug discovery
InactiveUS7070996B2BiocideMicrobiological testing/measurementProgenitorHigh-Throughput Screening Methods
Provided are methods for producing and screening proliferated populations of CD34-negative progenitor cells, mucosal mast cells, connective tissue-type mast cells and basophil cells. The methods generate uniform proliferated populations of cells. The proliferated populations contain a uniform population of a size suitable for use in high throughput screening methods, for example, screening for agents that alter exocytosis. The invention includes screening the proliferated populations with at least one candidate bioactive agent, and evaluating the cells to detect a cell with an altered phenotype. The invention also includes isolating a candidate bioactive agent that causes the altered phenotype. Additionally, cells formed according to the described methods are also encompassed by the invention.
Owner:RIGEL PHARMA
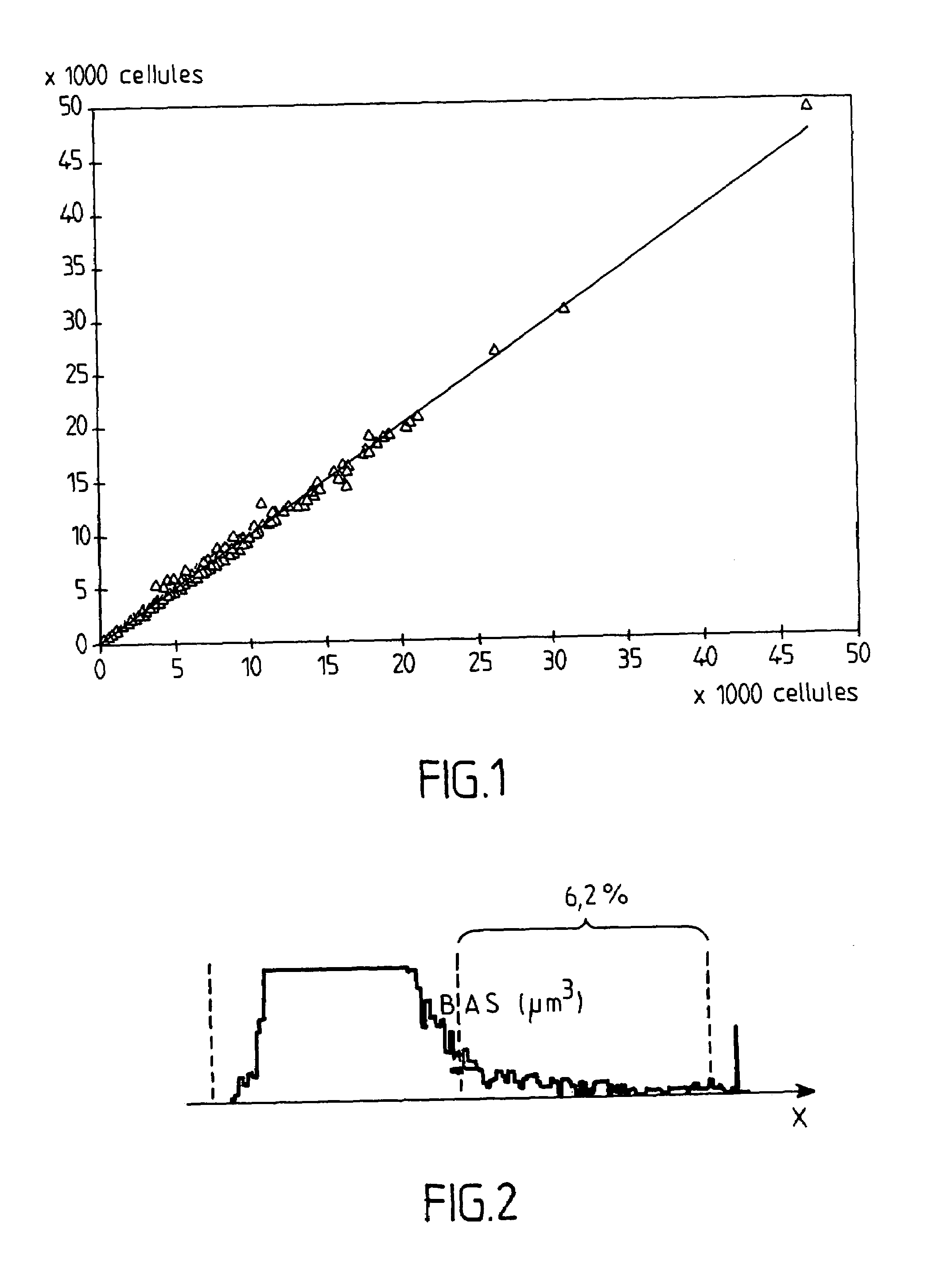
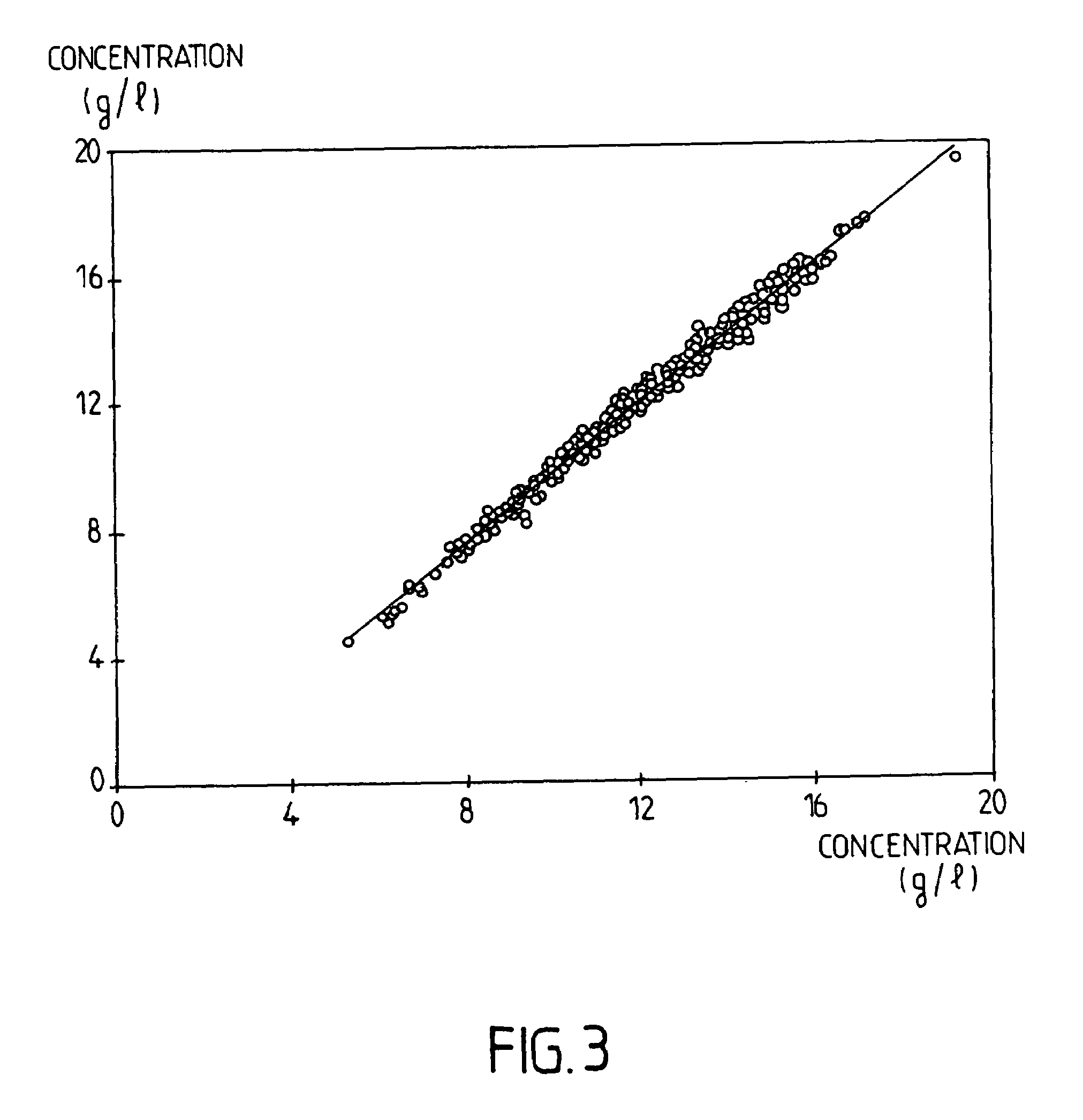
Reagent for determination of leucocytes and measurement of haemoglobin in a sample of blood
The invention concerns a reagent for determination of leucocytes and measurement of haemoglobin in a sample of blood. This reagent comprises a buffer system that is suited to adjust selectively the pH of the reagent to an acidic value, at least one detergent of cationic type, a nitrogenous compound and, optionally, at least one inorganic salt. This reagent can be used in haematological analyses in human medicine and also permits the identification of a leucocytic subpopulation, in particular the basophil polymorphonuclear leucocytes.
Owner:HORIBA ABX SAS
Integrated and standalone label and reagent-free microfluidic devices and microsystems for differential white blood cell counts
InactiveUS20160274020A1Eliminate needImprove signal-to-noise ratioMaterial analysis by electric/magnetic meansBiological particle analysisCell freeNeutrophil granulocyte
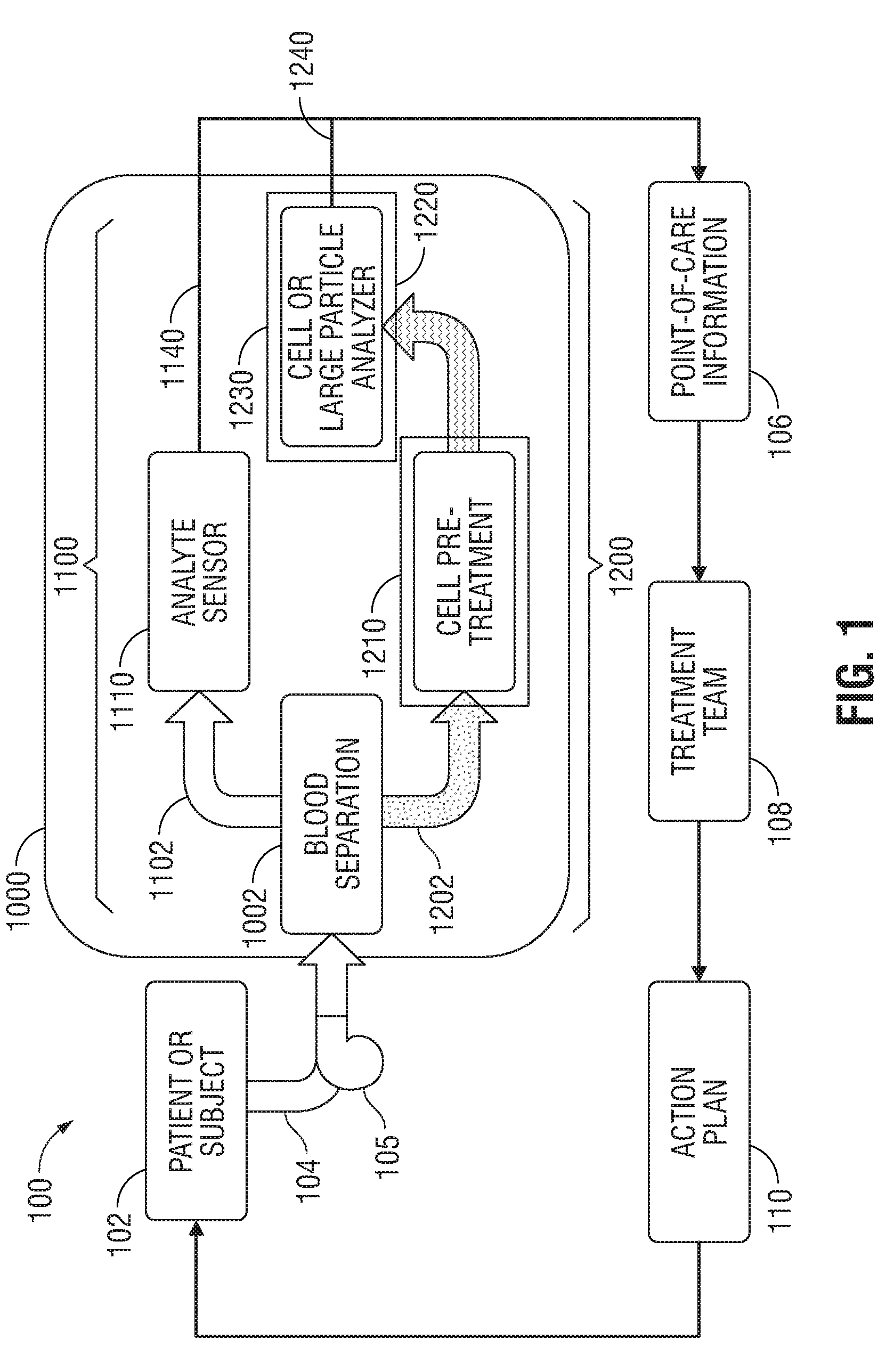
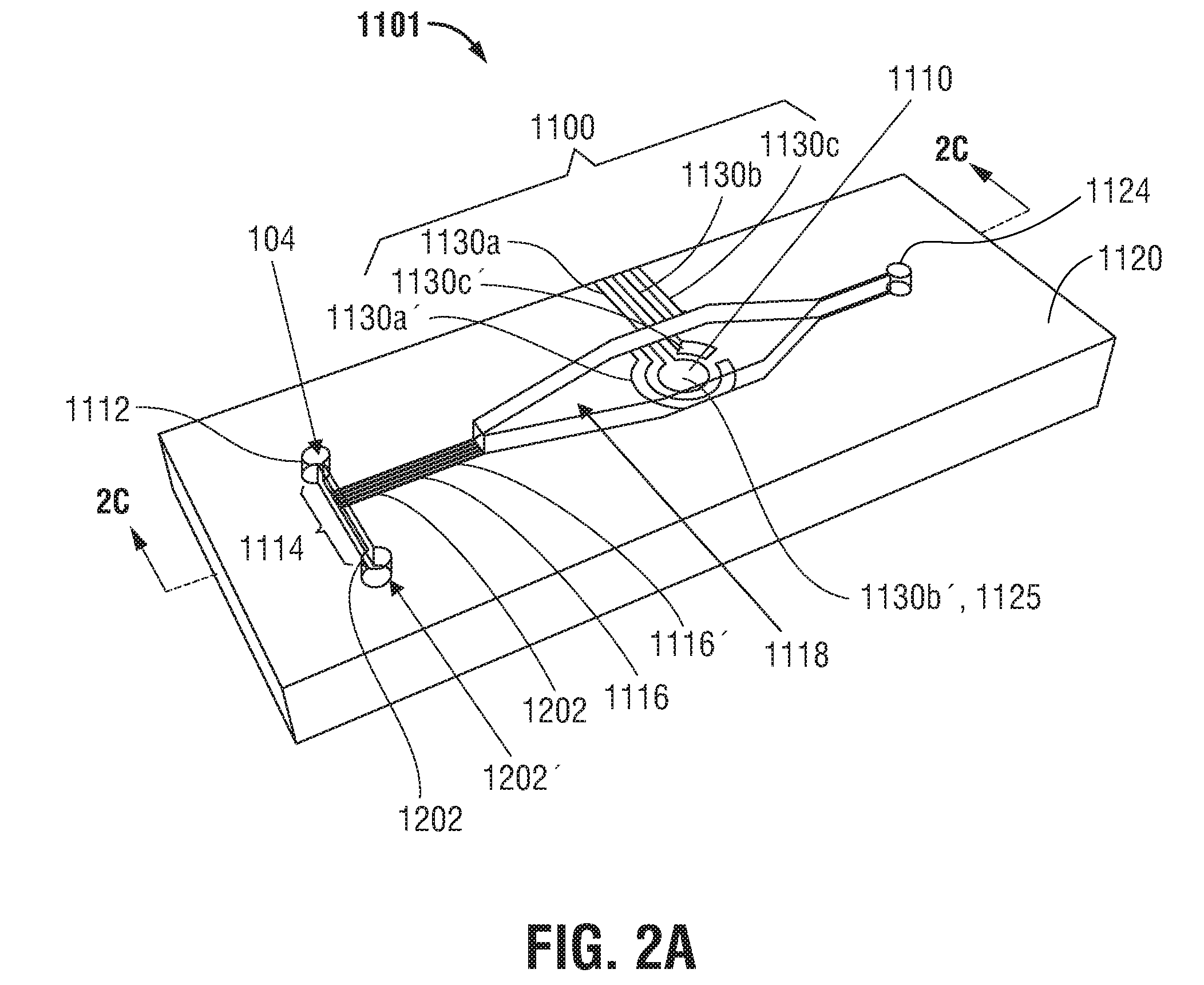
A method of establishing a differential white blood cell count includes directing at least one stream of deionized water into a microfluidic device containing a sample of whole blood or a cell-rich fraction to generate a lysate stream of intact white blood cells; directing at least one stream of deionized water into the lysate stream to form a virtual non-conductive aperture in a channel of the device; and performing impedance cytometry of the lysate stream via coplanar electrodes to detect the presence of intact white blood cells. A microfluidic device includes a blood separation section. An analyte sensor detects electrical changes in a cell-free fraction. Lysate from a cell-rich fraction is analyzed to detect circulating tumor cells or white blood cells including neutrophils, lymphocytes, monocytes, eosinophils, and basophils. A method of fabricating and a standalone cell-rich microfluidic device are disclosed for differential white blood cell counts.
Owner:UNIV OF MARYLAND
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS20030059912A1Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsADAMTS ProteinsPotassium
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-Ile-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Allergen vaccine proteins for the treatment and prevention of allergic diseases
InactiveUS7566456B2Ameliorating and reducing risk of IgE-medicatedReduce riskPeptide/protein ingredientsAntibody mimetics/scaffoldsFc(alpha) receptorCross-link
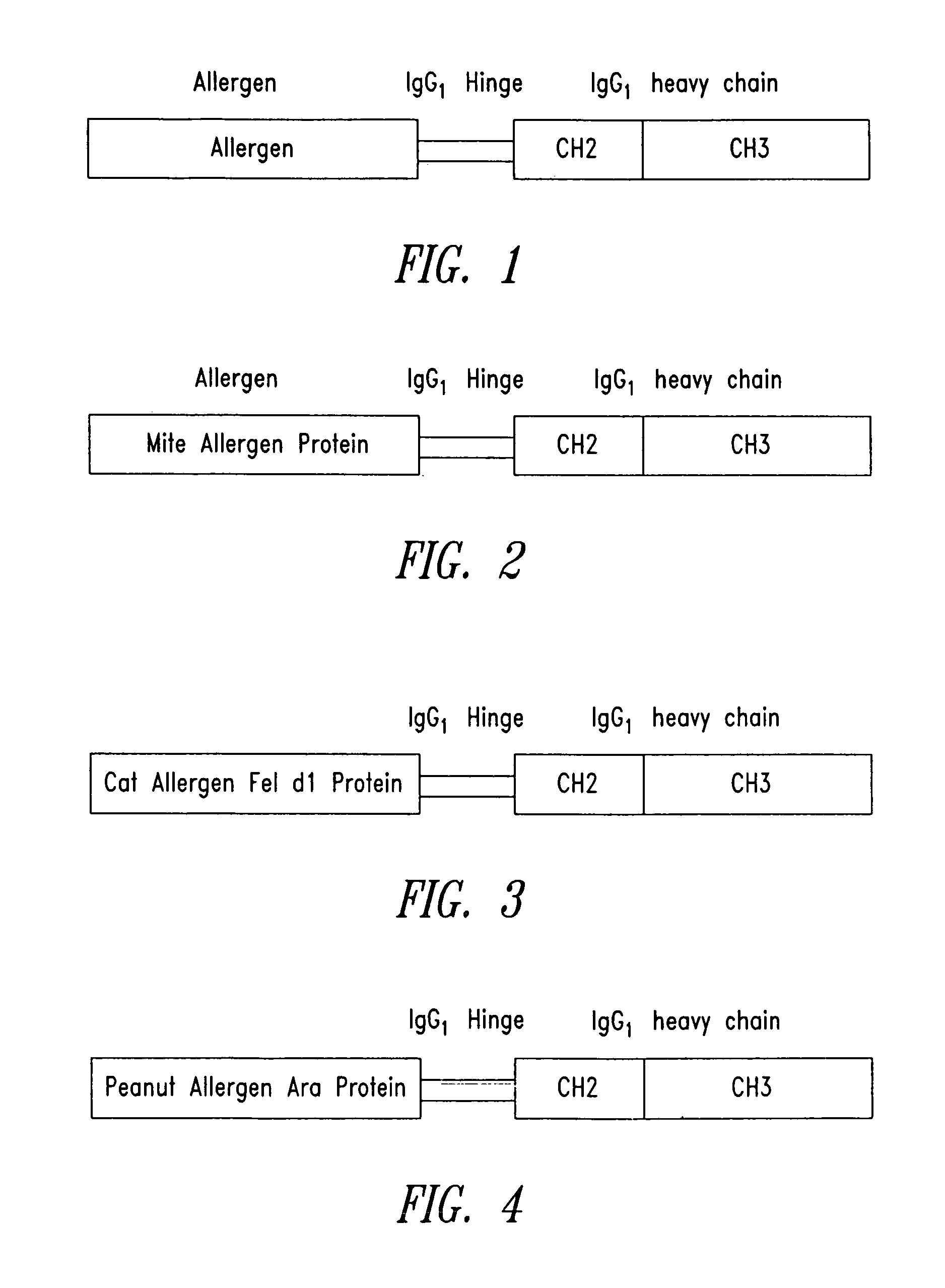
The present invention provides fusion proteins comprising an allergen sequence linked via an IgG hinge region to another polypeptide sequence capable of specifically binding to a native IgG inhibitory receptor containing an immune receptor tyrosine based inhibitory motif (ITIM). They are designed to cross-link an Fc receptor for IgE (e.g., FcεR1) and an IgG inhibitory receptor (e.g., FcγRIIb), thereby inhibiting the IgE-driven mediators released from mast cells and basophils. In addition, the present invention provides nucleic acid molecules encoding the fusion proteins, vectors and host cells for producing the fusion proteins, pharmaceutical compositions comprising the fusion proteins, and methods for ameliorating or reducing the risk of IgE-medicated allergic diseases.
Owner:CHEN HAIMING
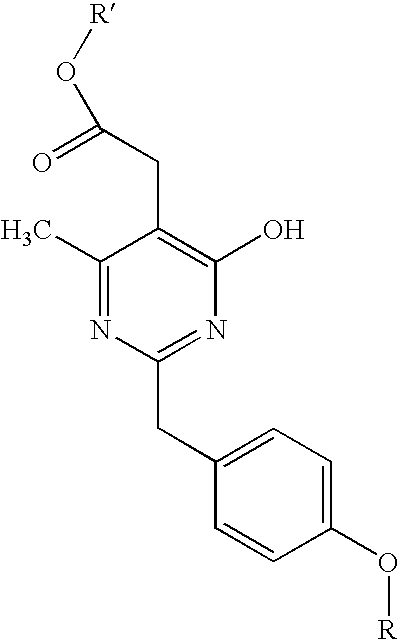
Pyrimidine derivatives useful for the treatment of diseases mediated by crth2
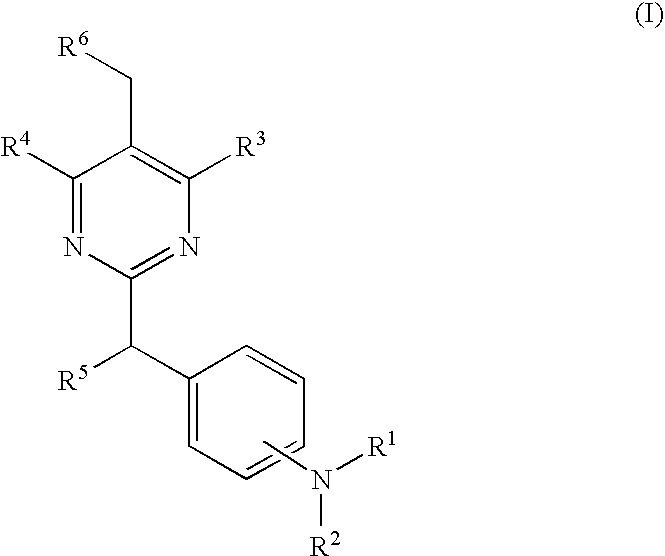
The present invention relates to a pyrimidine derivative of the formula (I) and salts thereof which is useful as an active ingredient of pharmaceutical preparations. The pyrimidine derivative of the present invention has excellent CRTH2 (G-protein-coupled chemoattractant receptor, expressed on Th2 cells) antagonistic activity and can be used for the prophylaxis and treatment of diseases associated with CRTH2 activity, in particular for the treatment of allergic diseases, such as asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis; eosinophil-related diseases, such as Churg-Strauss syndrome and sinusitis; basophil-related diseases, such as basophilic leukemia, chronic urticaria and basophilic leukocytosis in human and other mammals; and inflammatory diseases characterized by T lymphocytes and profuse leukocyte infiltrates such as psoriasis, eczema, inflammatory bowel disease, ulcerative colitis, Crohn's disease, COPD (chronic obstructive pulmonary disorder) and arthritis.
Owner:GB007 INC
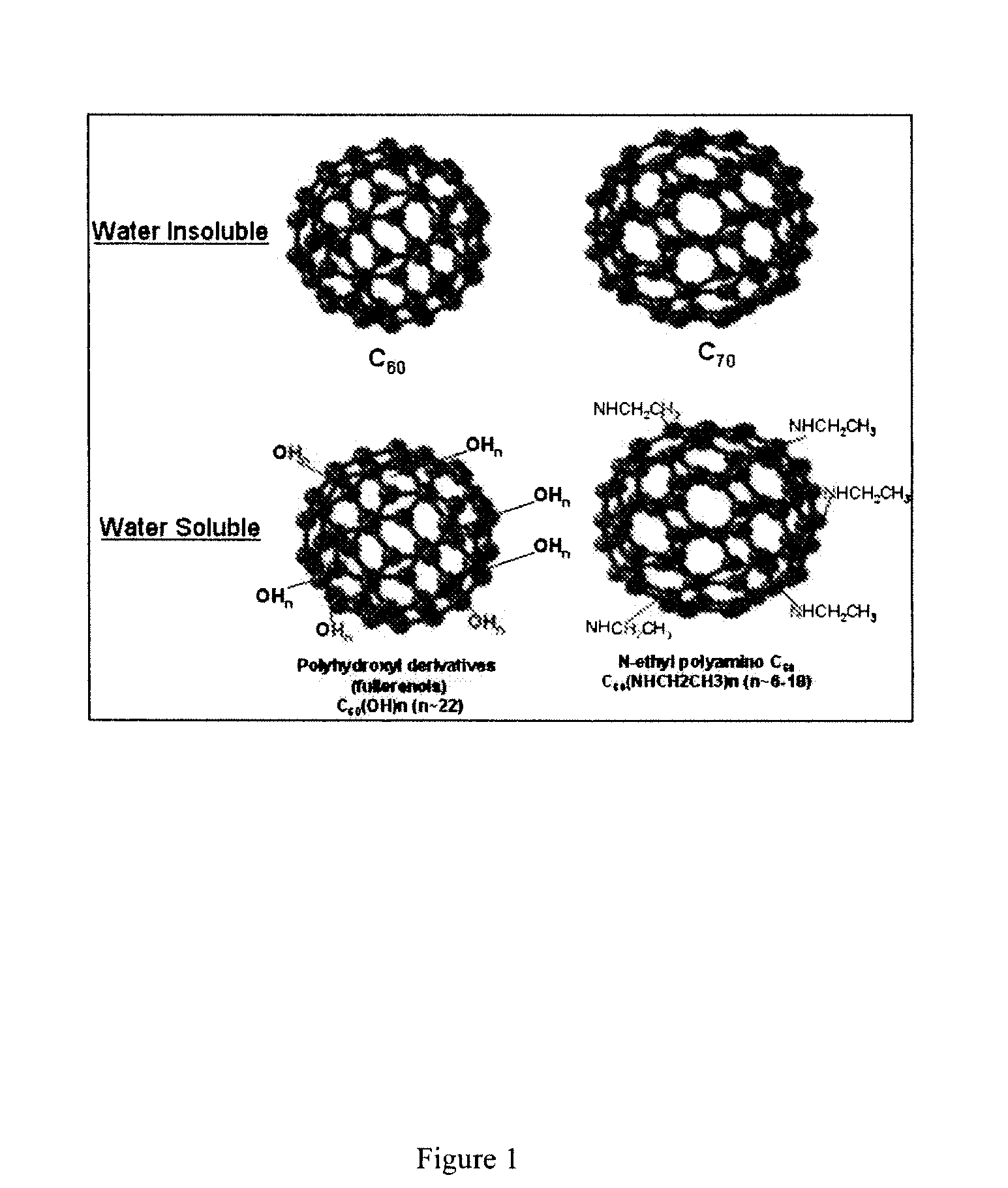
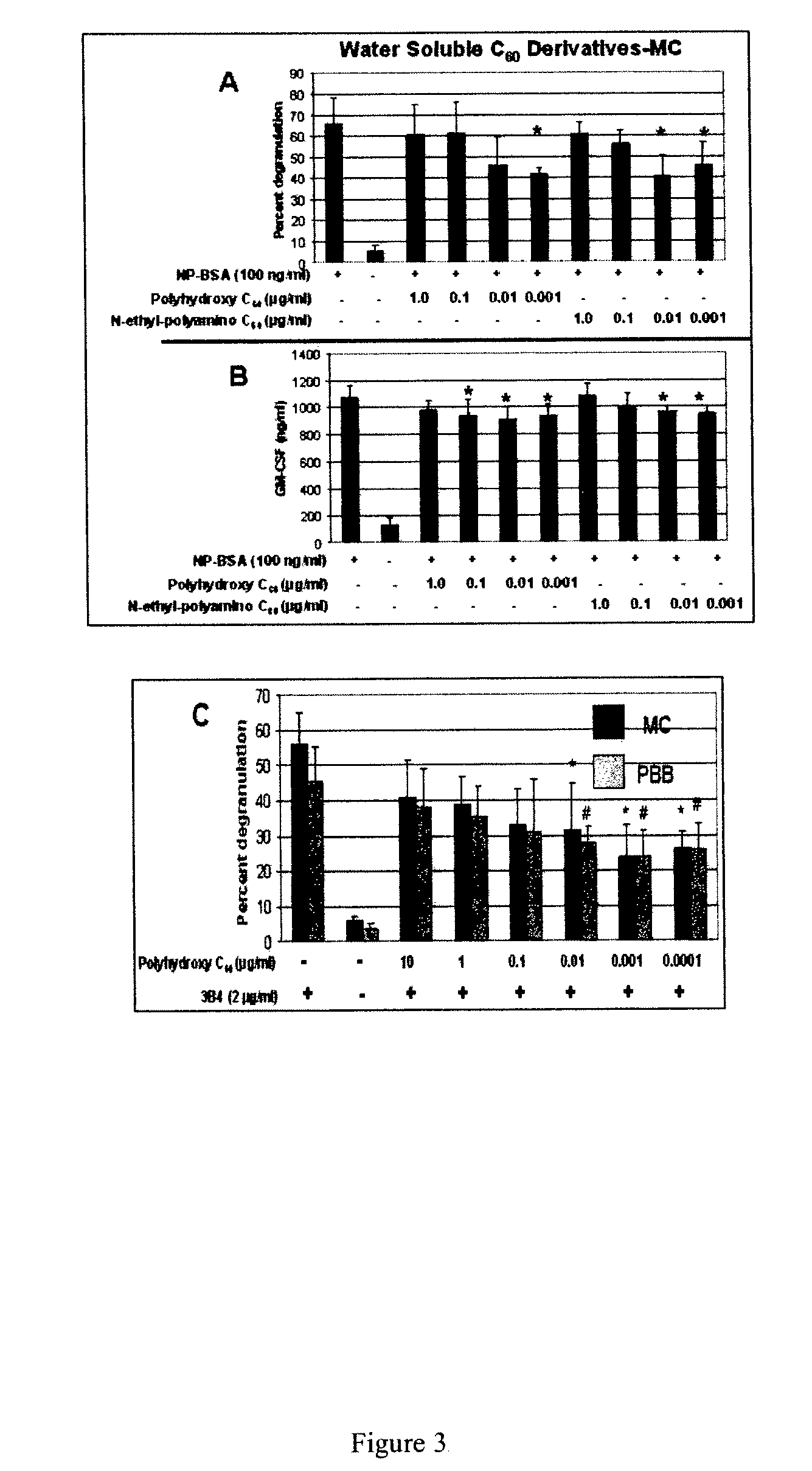
Use of fullerenes for the treatment of mast cell and basophil-mediated disease
InactiveUS20080107618A1Lower Level RequirementsGood water solubilityBiocideHydroxy compound active ingredientsDiseaseAntigen
Mast cell (MC) and peripheral blood basophil (PBB)-associated diseases are treated or prevented, or their symptoms are alleviated by the administration of water soluble fullerenes (buckeyballs) to the individual under conditions sufficient to inhibit MC and PBB responses. MC and PBB responses are associated with, for example, various allergies including Type 1 hypersensitivity initiated by IgE-antigen, arthritis, multiple sclerosis, urticaria, atopic dermatitis, heart disease, etc. The treatment regimen can be enhanced using Chimeric fullerenes that specifically home to and inhibit MC and PBB cells. These molecules, for example, comprise fullerenes to which are attached IgE Fc or stem cell factor (SCF) peptides that bind to receptors specifically on MC and PBB cells. Additional molecules which may be used in the processes include IgE Fc or SCF peptides with several fullerenes covalently attached.
Owner:VIRGINIA COMMONWEALTH UNIV
Method for binding basophils and mast cells
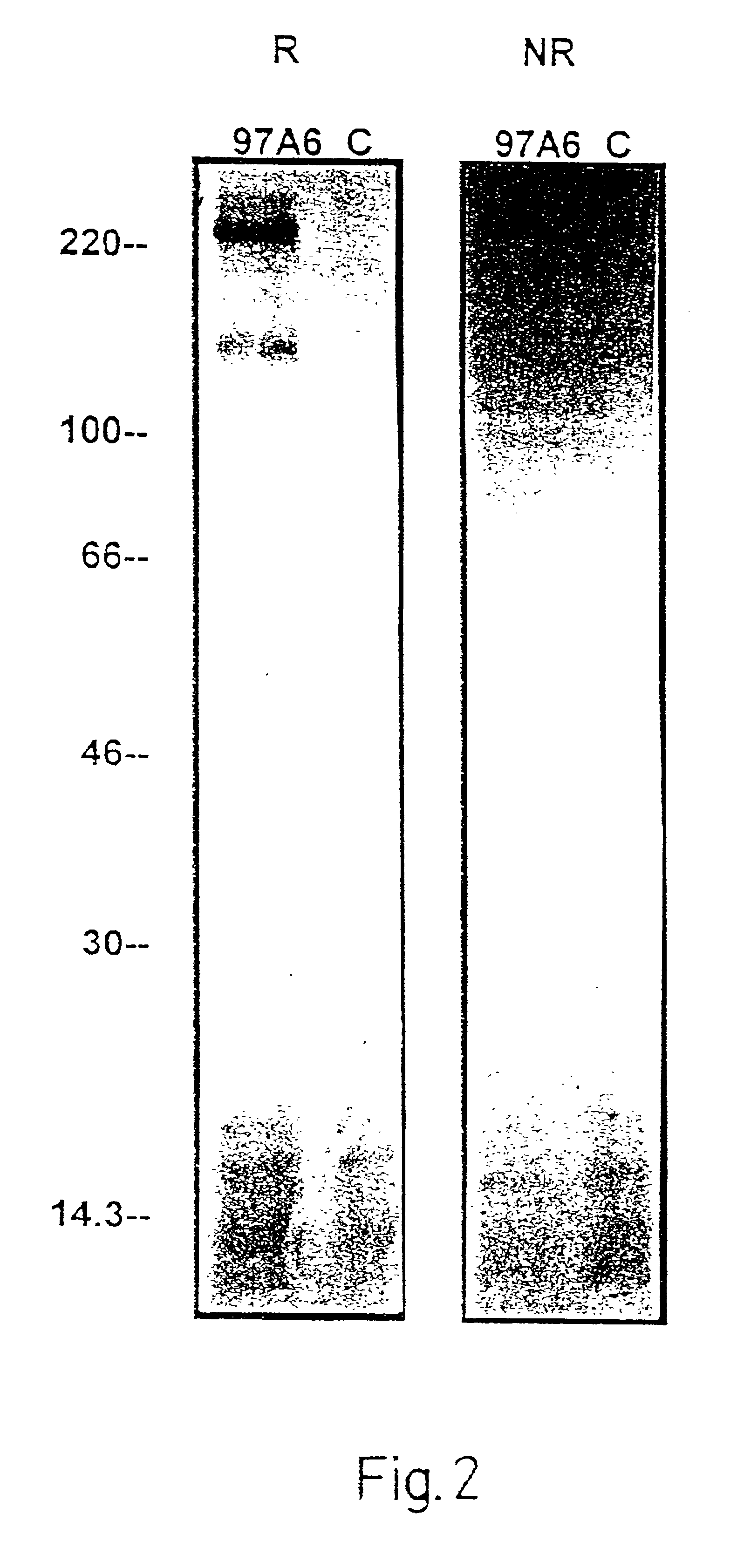
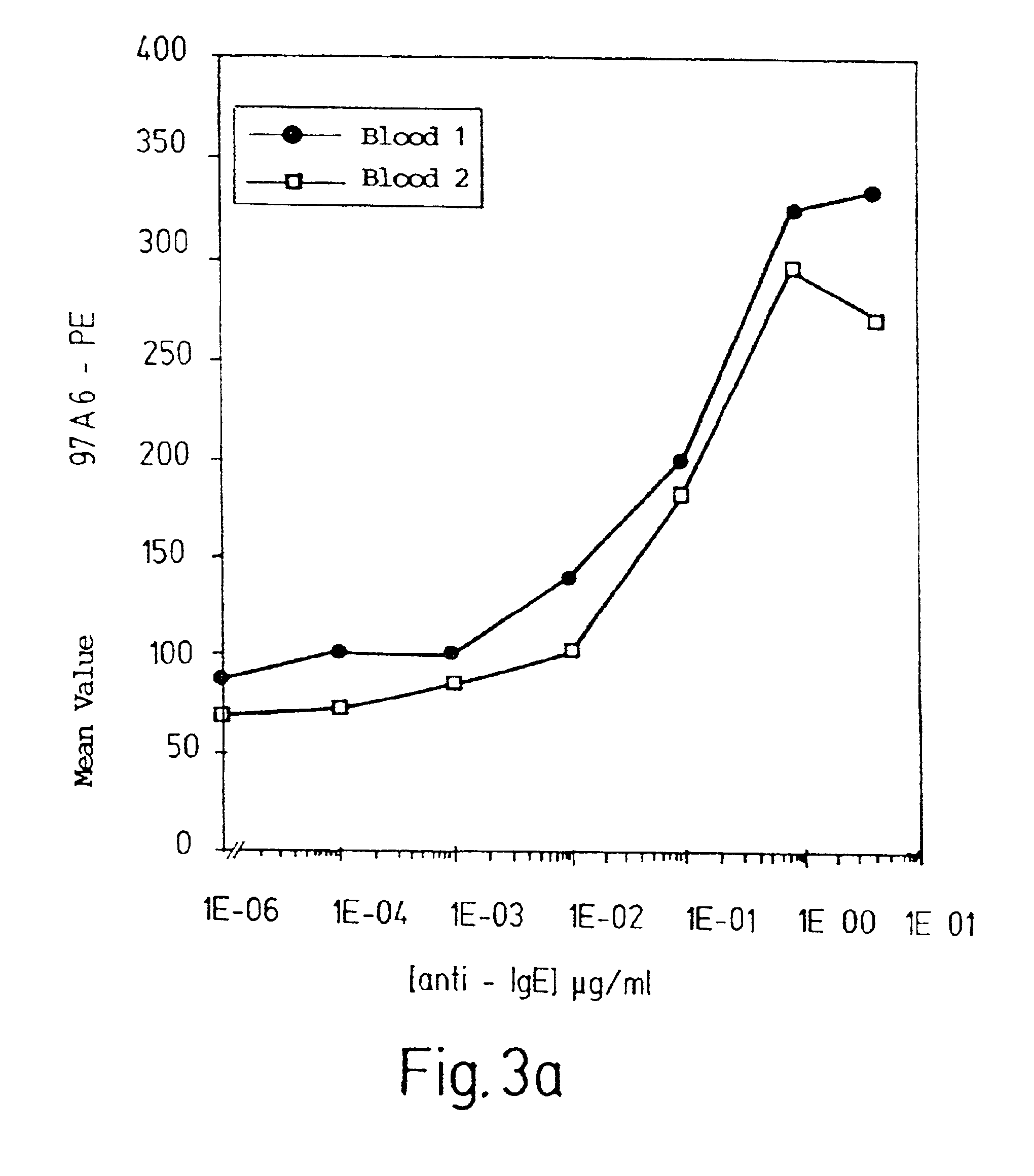
InactiveUS6949346B2Improve isolationCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsMast cellBasophil
The present invention relates to an antibody for the detection quantification, or isolation of basophils, mast cells, the precursor cells of basophils or mast cells, or a surface structure of basophils or mast cells. This antibody corresponds to an antibody with the designation 97A6, produced and released by hybridoma cells that were deposited in accordance with the Budapest Treaty on Feb. 12, 1997 under accession number DSM ACC 2297 at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ).
Owner:UNIV TUBINGEN
Allergen vaccine proteins for the treatment and prevention of allergic diseases
InactiveUS20060292138A1Reduce riskAmeliorating and reducing risk of IgE-medicatedPeptide/protein ingredientsAntibody mimetics/scaffoldsFc(alpha) receptorCross-link
The present invention provides fusion proteins comprising an allergen sequence linked via an IgG hinge region to another polypeptide sequence capable of specifically binding to a native IgG inhibitory receptor containing an immune receptor tyrosine based inhibitory motif (ITIM). They are designed to cross-link an Fc receptor for IgE (e.g., FcεR1) and an IgG inhibitory receptor (e.g., FcγRIIb), thereby inhibiting the IgE-driven mediators released from mast cells and basophils. In addition, the present invention provides nucleic acid molecules encoding the fusion proteins, vectors and host cells for producing the fusion proteins, pharmaceutical compositions comprising the fusion proteins, and methods for ameliorating or reducing the risk of IgE-medicated allergic diseases.
Owner:CHEN HAIMING
Composition comprising herbal extracts or fermented products thereof having lactic acid bacteria for preventing or treating respiratory diseases
ActiveUS20140140984A1Lower the resistance valueReduce in quantityBiocideUnknown materialsGoniothalamusDisease
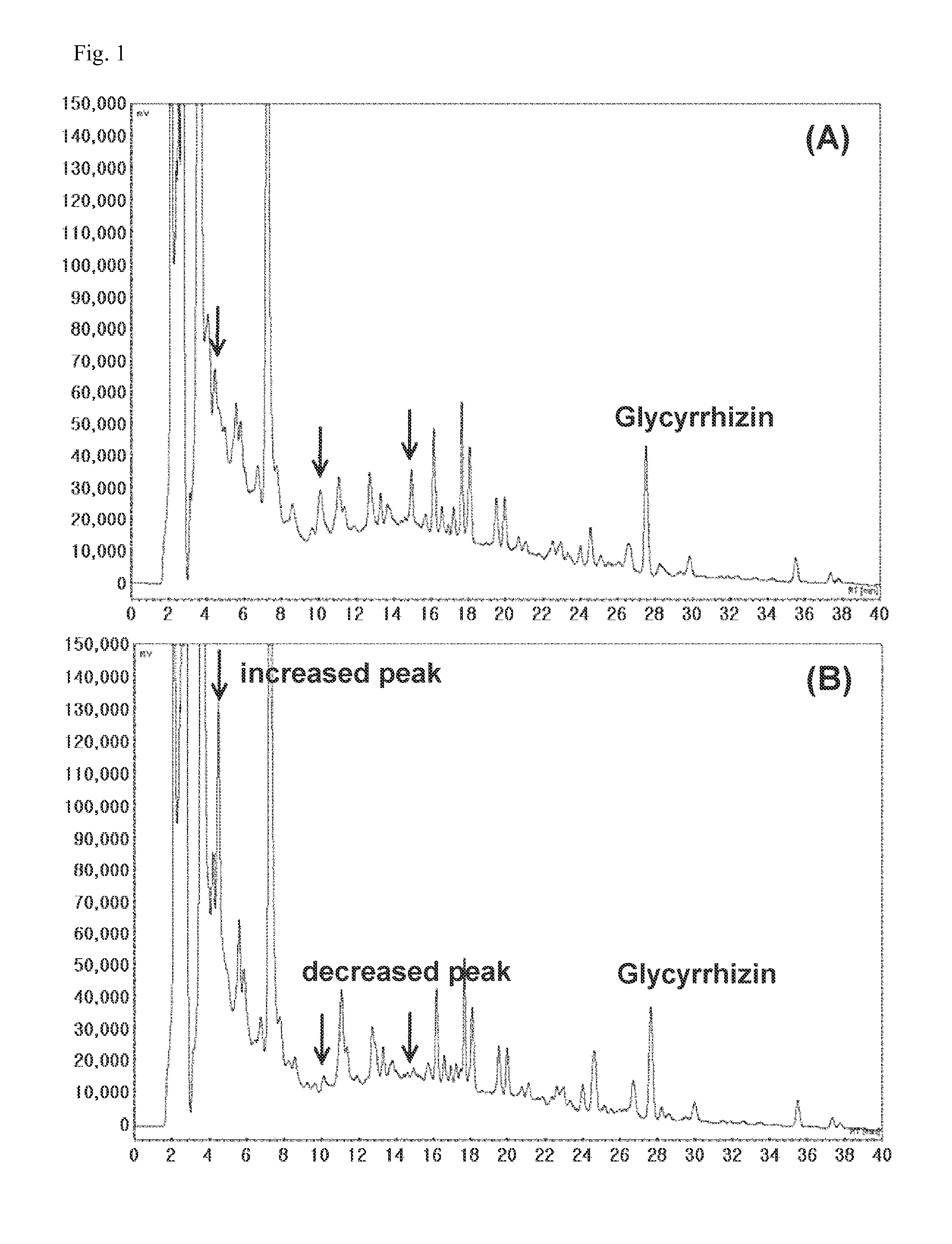
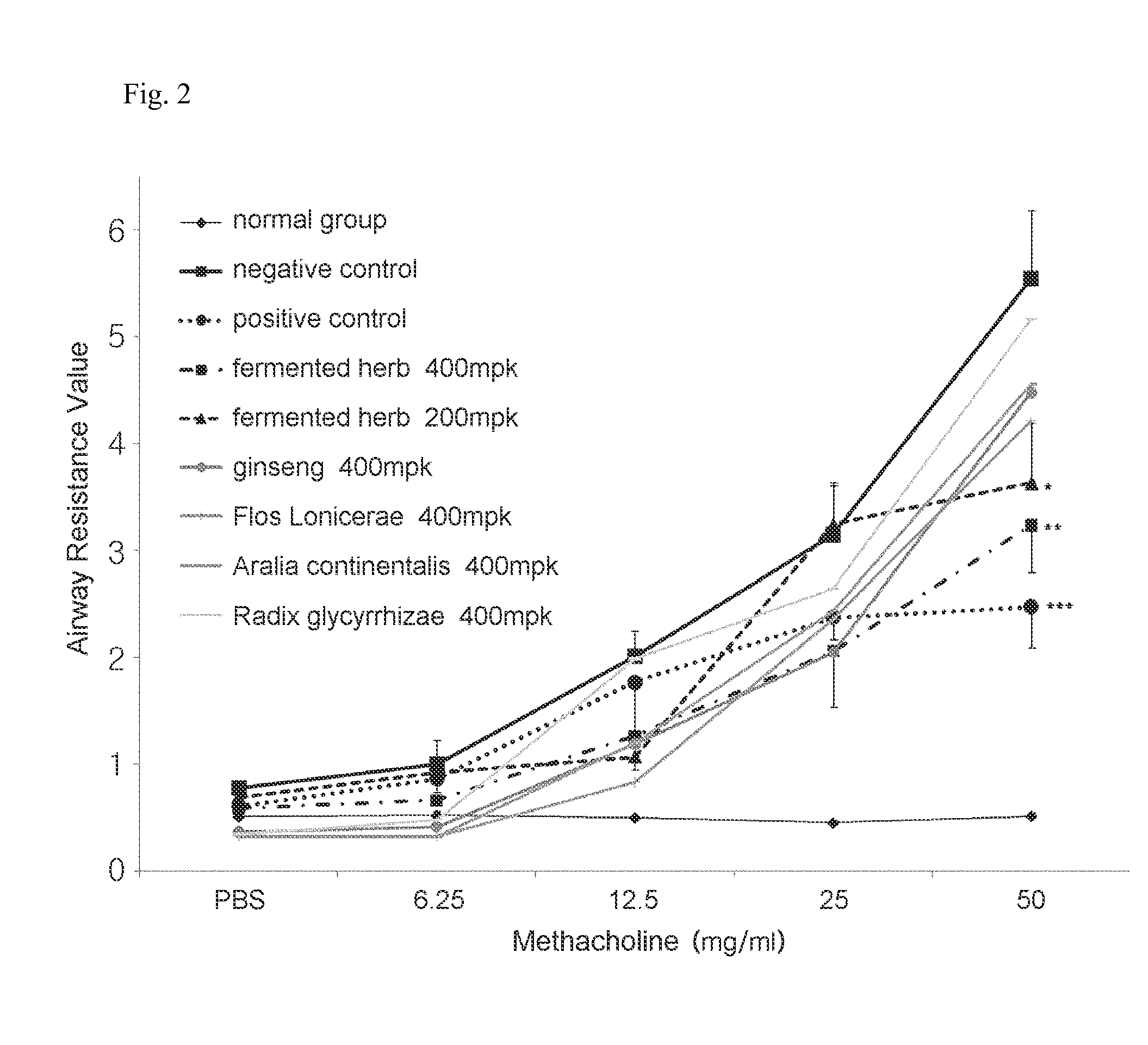
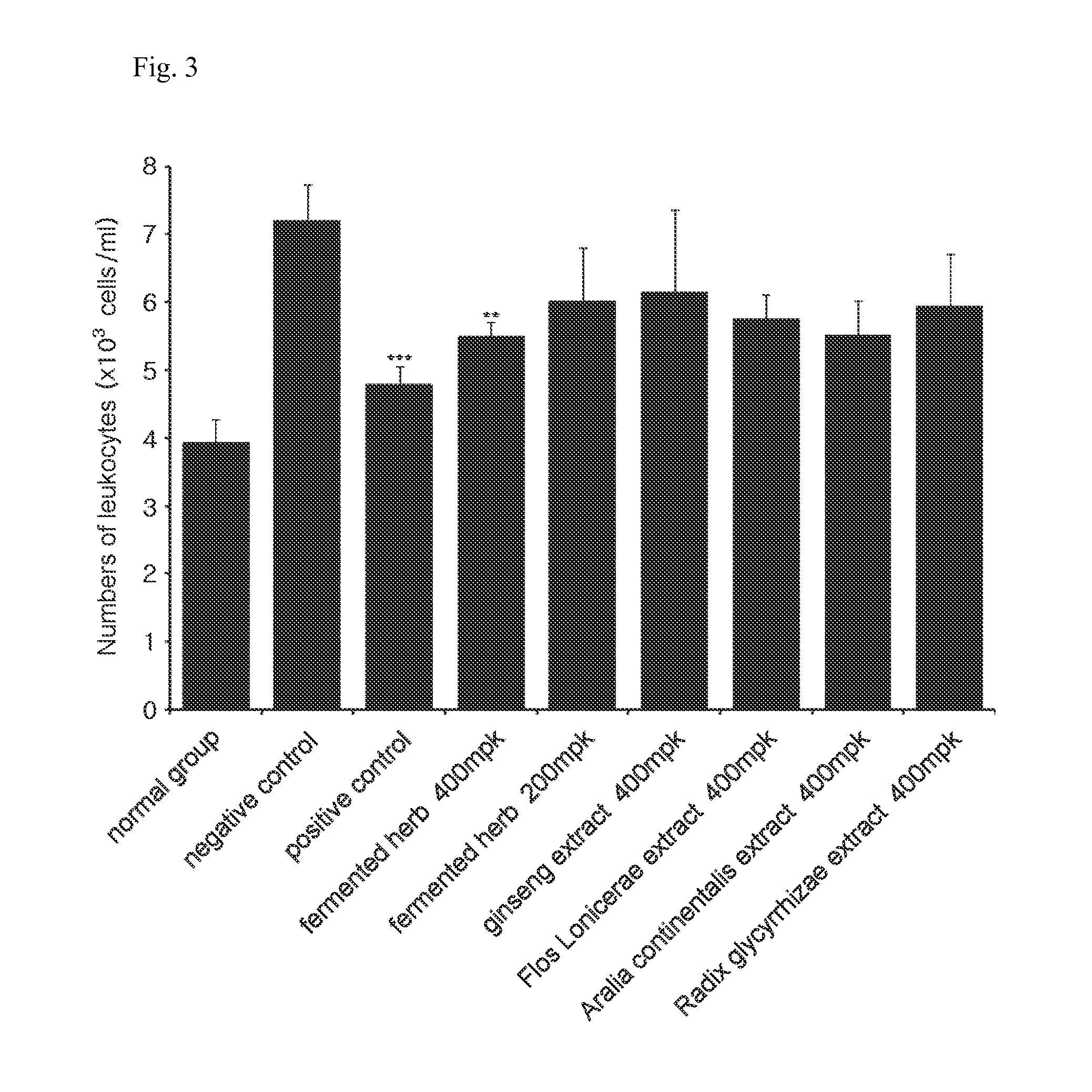
The present invention relates to a composition for preventing or treating respiratory diseases, and more particularly, to a pharmaceutical composition, and to a health food, comprising extracts of herbal mixtures or fermented products thereof having lactic acid bacteria as effective ingredients for preventing or treating respiratory diseases, wherein the herbal mixtures comprise: Sophora flavascens, Radix glycyrrhizae, Flos Lonicerae, Angelicae Gigantis radix, Aralia continentalis, Inula helenium, Saposhnikoviae radix, Zizyphus spinosa, Houttuynia cordata, Forsythiae fructus, Arctium lappa, Herba epimedii, ginseng, Lithospermi radix, Sanguisorbae radix, Cnidii rhizoma, Scrophulariae radix, and Polygoni cuspidati radix. The herbal extracts or fermented products thereof having lactic acid bacteria exhibit the effects of significantly lowering an airway resistance value; reducing the numbers of leukocytes, neutrophilic leukocytes, lymphocytes, eosinocytes, and basophil leukocytes to levels similar to that of a normal group; and reducing the infiltration of inflammatory cells and eosinocyte cells near AHR on lung tissues. Therefore, the herbal extracts or fermented products thereof having lactic acid bacteria may be valuably used for the prevention and / or treatment of respiratory diseases such as asthma.
Owner:KOREA INST OF ORIENTAL MEDICINE
Production of cultured human mast cells and basophils for high throughput small molecule drug discovery
Provided are methods for producing and screening proliferated populations of CD34-negative progenitor cells, mucosal mast cells, connective tissue-type mast cells and basophil cells. The methods generate uniform proliferated populations of cells. The proliferated populations contain a uniform population of a size suitable for use in high throughput screening methods, for example, screening for agents that alter exocytosis. The invention includes screening the proliferated populations with at least one candidate bioactive agent, and evaluating the cells to detect a cell with an altered phenotype. The invention also includes isolating a candidate bioactive agent that causes the altered phenotype. Additionally, cells formed according to the described methods are also encompassed by the invention.
Owner:RIGEL PHARMA
Pyrimidine derivatives useful for the treatment of diseases mediated by CRTH2
InactiveUS7812160B2Excellent CRTH antagonistic activityGood choiceBiocideOrganic active ingredientsSinusitisWhite blood cell
The present invention relates to a pyrimidine derivative of the formula (I) and salts thereof which is useful as an active ingredient of pharmaceutical preparations. The pyrimidine derivative of the present invention has excellent CRTH2 (G-protein-coupled chemoattractant receptor, expressed on Th2 cells) antagonistic activity and can be used for the prophylaxis and treatment of diseases associated with CRTH2 activity, in particular for the treatment of allergic diseases, such as asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis; eosinophil-related diseases, such as Churg-Strauss syndrome and sinusitis; basophil-related diseases, such as basophilic leukemia, chronic urticaria and basophilic leukocytosis in human and other mammals; and inflammatory diseases characterized by T lymphocytes and profuse leukocyte infiltrates such as psoriasis, eczema, inflammatory bowel disease, ulcerative colitis, Crohn's disease, COPD (chronic obstructive pulmonary disorder) and arthritis.
Owner:GB007 INC
Basophil analysis system and method
InactiveUS9091625B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSub populationsFluorescence
Provided herein are systems and methods for analyzing blood samples, and more specifically for performing a basophil analysis. In one embodiment, the systems and methods include: (a) staining a blood sample with an exclusive cell membrane permeable fluorescent dye; and then (b) using measurements of light scatter and fluorescence emission to distinguish basophils from other WBC sub-populations. In one embodiment, the systems and methods include performing a basophil cluster analysis of the blood sample, based on the combination of light scatter and fluorescence measurements.
Owner:ABBOTT LAB INC
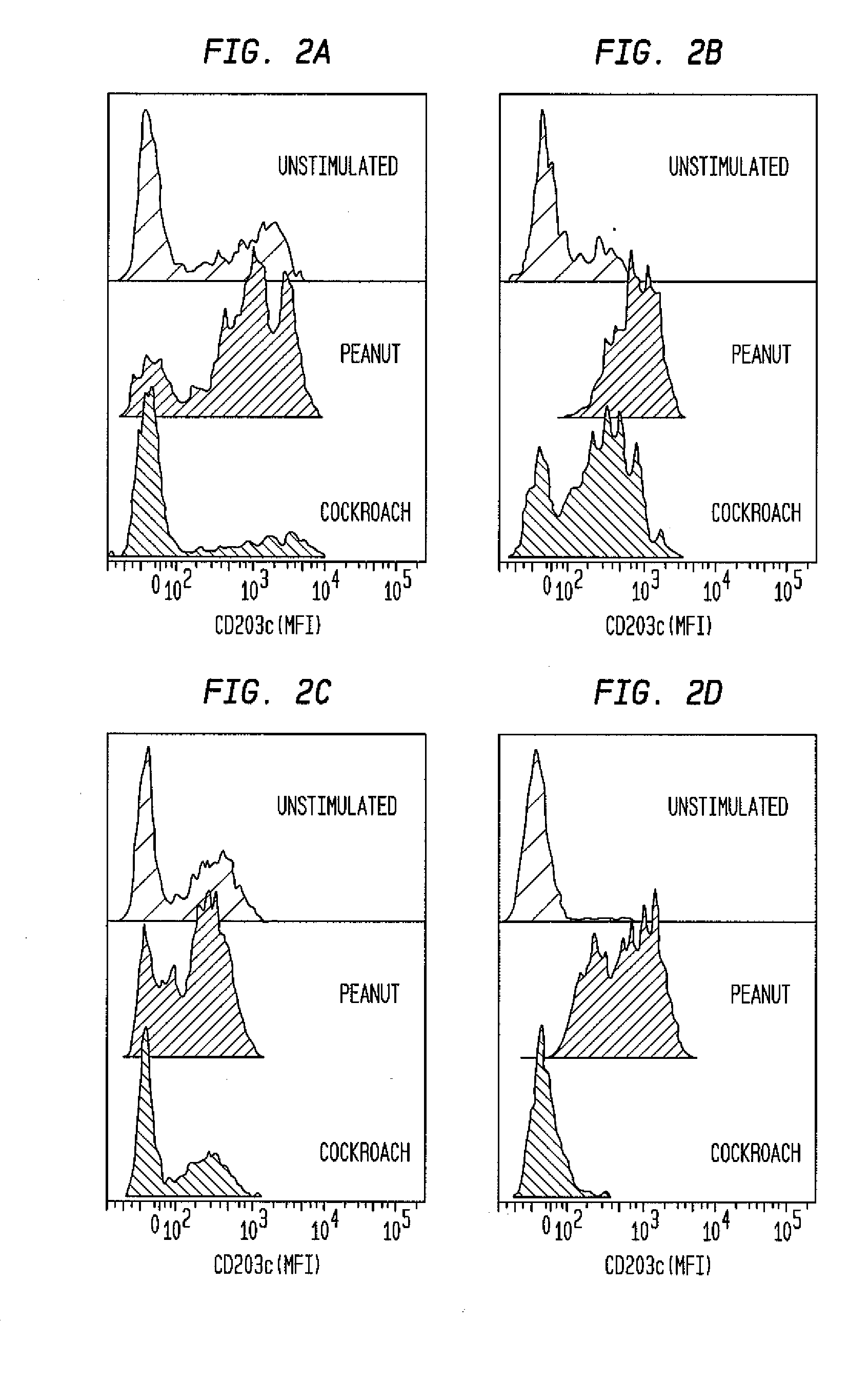
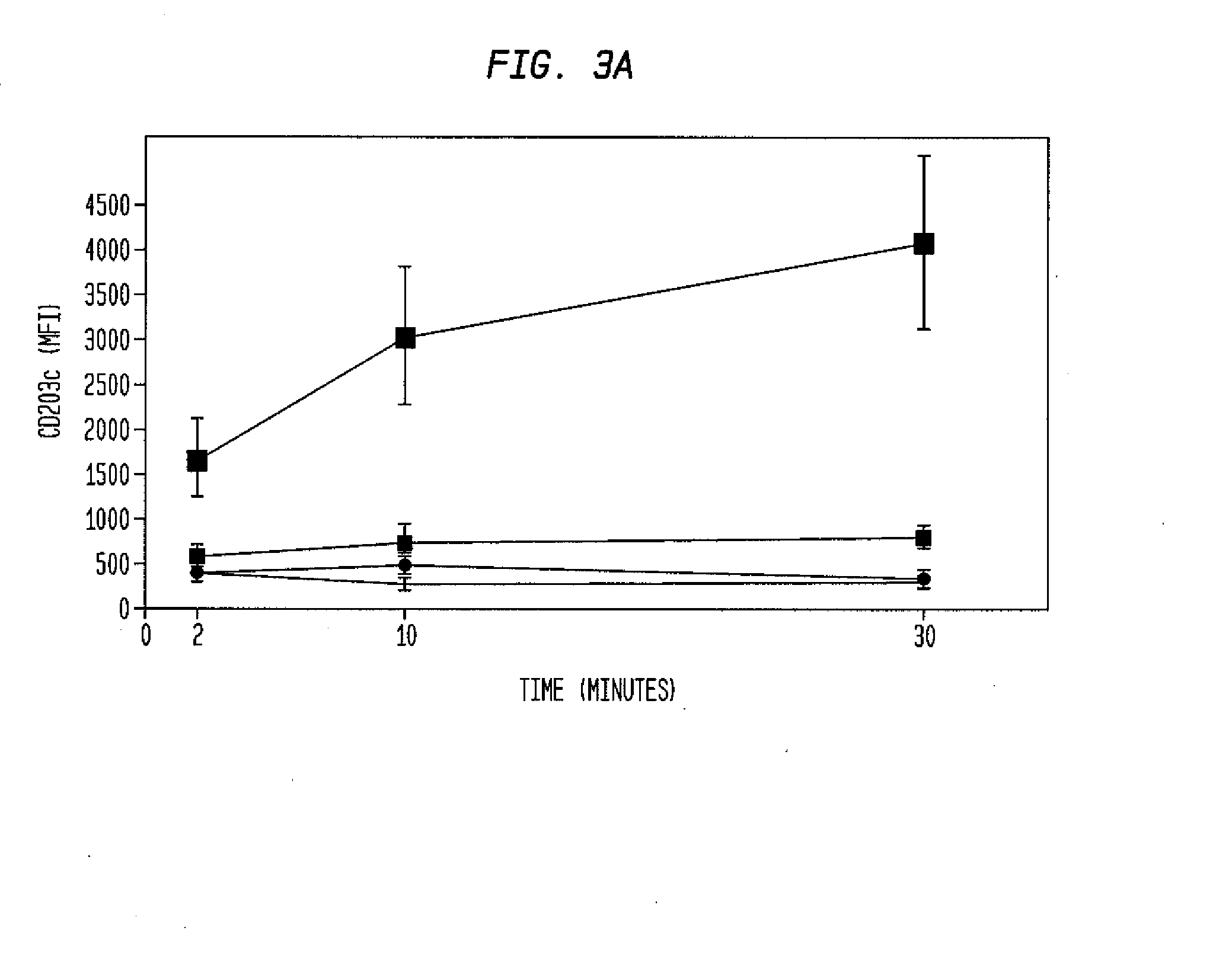
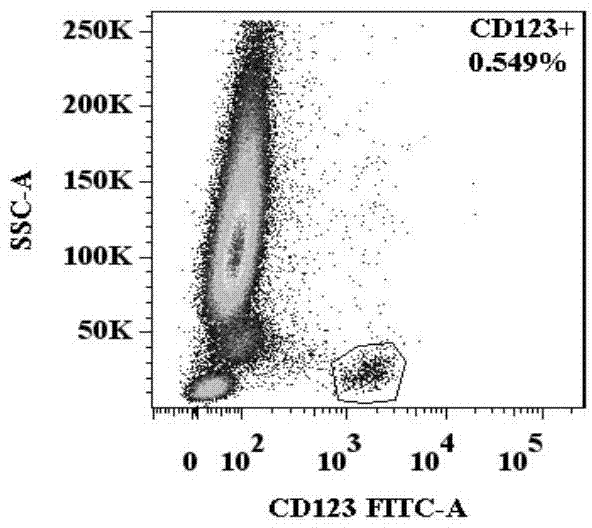
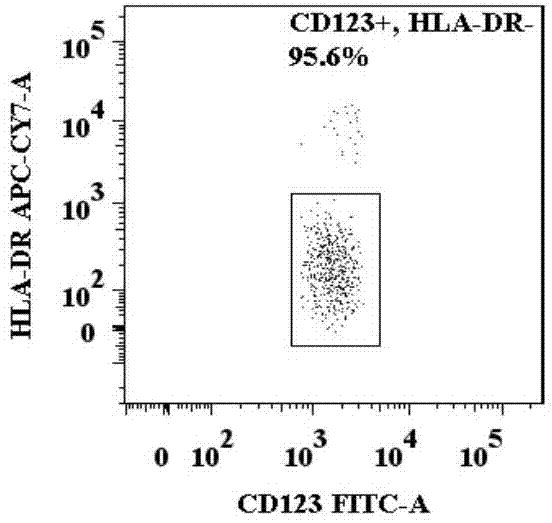
Basophilic granulocyte activation and degranulation identification method
ActiveCN104777303AGood repeatabilityReduce mistakesDisease diagnosisIndividual particle analysisRed blood cellTest sample
The invention provides a basophilic granulocyte activation and degranulation identification method. The basophilic granulocyte activation and degranulation identification method comprises the following steps that test samples are numbered in sequence, and flow type sample feeding pipes required by testing are marked; a required anti-human CD123, anti-human CCR3, anti-human HLA-DR, anti-human CD203c and / or anti-human CD63 fluorescence labeling flow type antibody combination is added into each flow type sample feeding pipe; mixed whole blood is added into the marked flow type pipes, and shading incubation is conducted at the indoor temperature; red blood cell lysate is added into each sample pipe, and shading incubation is conducted at the indoor temperature again; supernatant is removed in a centrifugal mode after incubation; detection is conducted by means of a flow cytometry, and the number of basophilic granulocytes and the average fluorescence intensity are analyzed. According to the basophilic granulocyte activation and degranulation identification method, 80%-100% of the basophilic granulocytes in peripheral blood can be distinguished from other types of cells under the condition that the basophilic granulocytes do not need to be separated or purified, the basophilic granulocyte activation and / or degranulation state can be identified, whole blood of no more than 100 microlitres is needed by each sample to be tested, and the repeatability is good.
Owner:HPY BIOTECH CO LTD
Pharmaceutical composition preparation
InactiveCN111467498AReduce exacerbationsImprove lung functionRespiratory disorderHeterocyclic compound active ingredientsAnticholinergic DrugsGlucocorticoid
The invention provides a pharmaceutical composition preparation. The pharmaceutical composition preparation comprises an anticholinergic drug tiotropium or a hydrate thereof, a beta 2-receptor agonistarformoterol or a salt thereof, and an inhaled glucocorticoid fluticasone or an ester derivative thereof; tiotropium bromide acts on a muscarinic receptor on bronchial smooth muscles, the cholinergiceffect of acetylcholine released by the tail ends of parasympathetic nerves can be inhibited, and muscular tension is blocked; the arformoterol acts on a beta 2-receptor on an airway smooth muscle cell membrane, so that the release of degranulation and media of mast cells and basophils is reduced, the permeability of capillaries is reduced, and swinging of airway epithelial cilia is increased; the fluticasone is an effective anti-inflammatory drug, and when the three drugs are combined for use, the three mechanisms jointly exert the bronchus relaxing effect and the anti-inflammatory effect; the application range of the triple therapy is wider on the important clinical indexes of reducing acute exacerbation of patients suffering from chronic obstructive pulmonary disease, reducing total-cause mortality rate, improving the lung function of the patients suffering from chronic obstructive pulmonary disease, improving the living quality and the like.
Owner:王兆霖
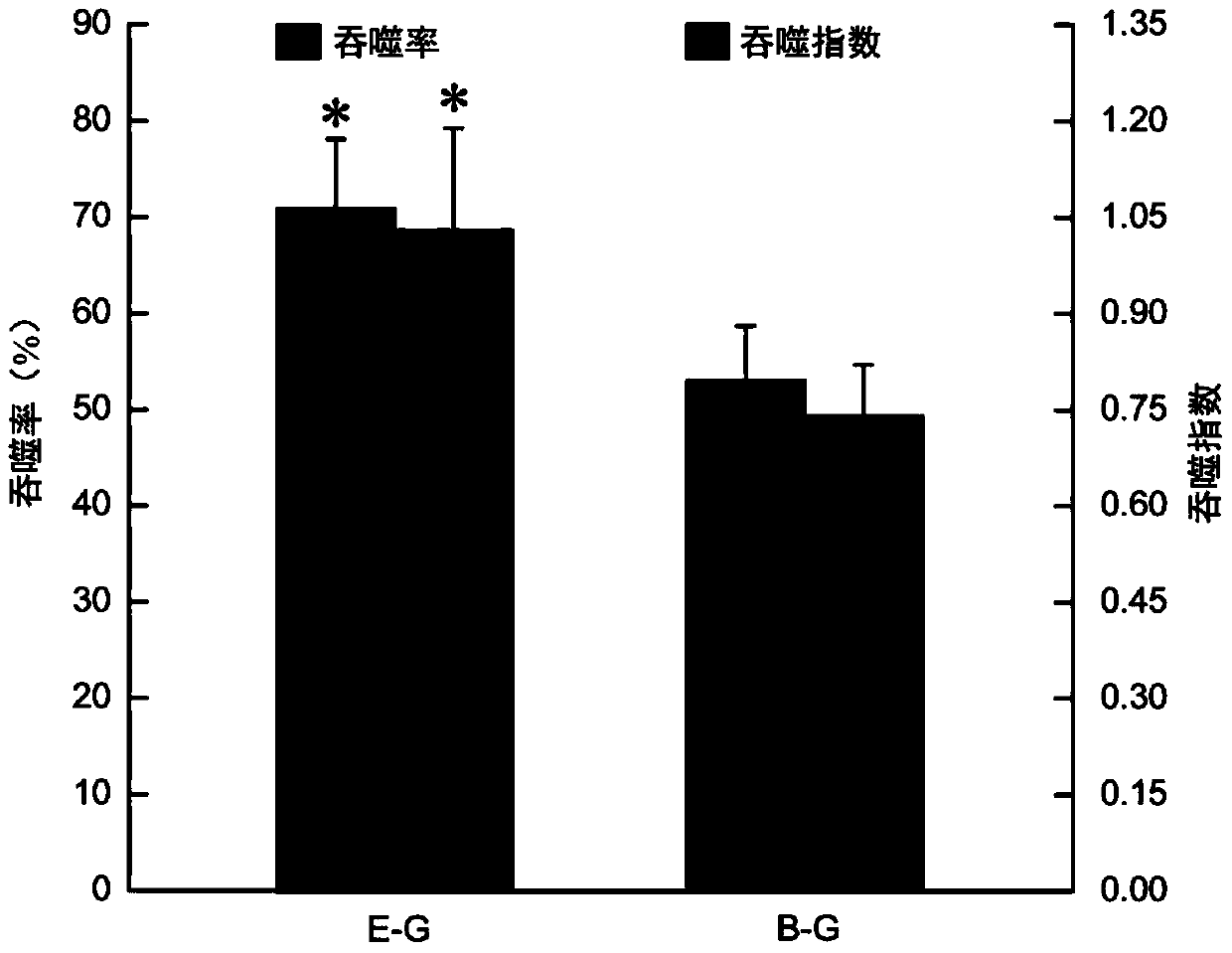
Method used for detecting ostrea plicatula eosinophilic and basophilic granulocyte phagocytic ability
InactiveCN103994963AEasy to operateAccurate operationPreparing sample for investigationIndividual particle analysisBasophilEosinophilic cell
The invention relates to a method used for detecting ostrea plicatula eosinophilic and basophilic granulocyte phagocytic ability, and the method comprises (1) ostrea plicatula blood cell suspension preparation; (2) fluorescent microsphere phagocytosis; (3) staining with neutral red; (4) lining dying with propidium iodide; and (5) microscopy. The method has the advantages of being simple in operation, rapid and accurate, strong in specificity, high in repeatability, and the like.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
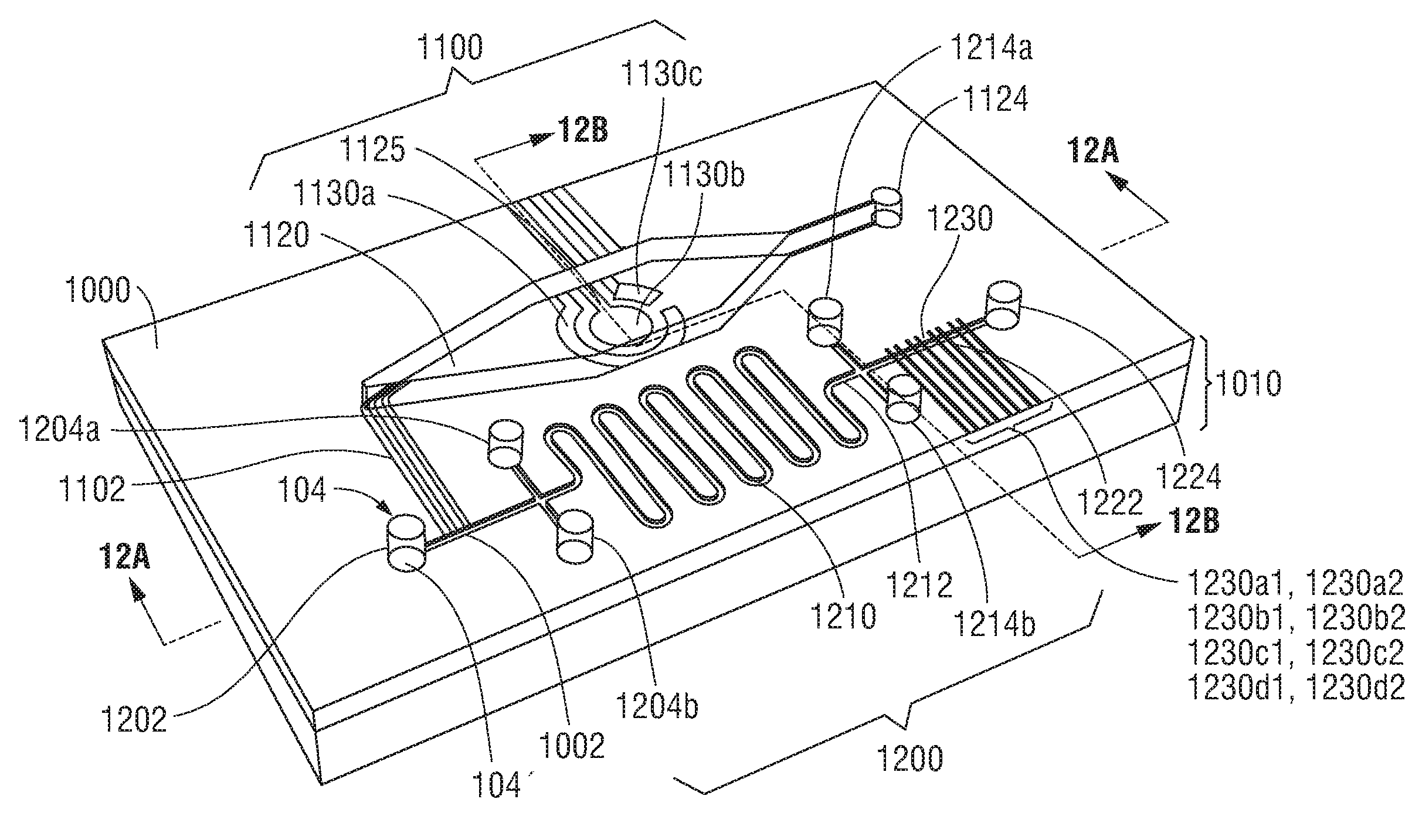
Process for enriching basophils in a blood sample
Owner:INST PASTEUR
Cell-specific signaling biomarker analysis by high parameter cytometry; sample processing, assay set-up, method, analysis
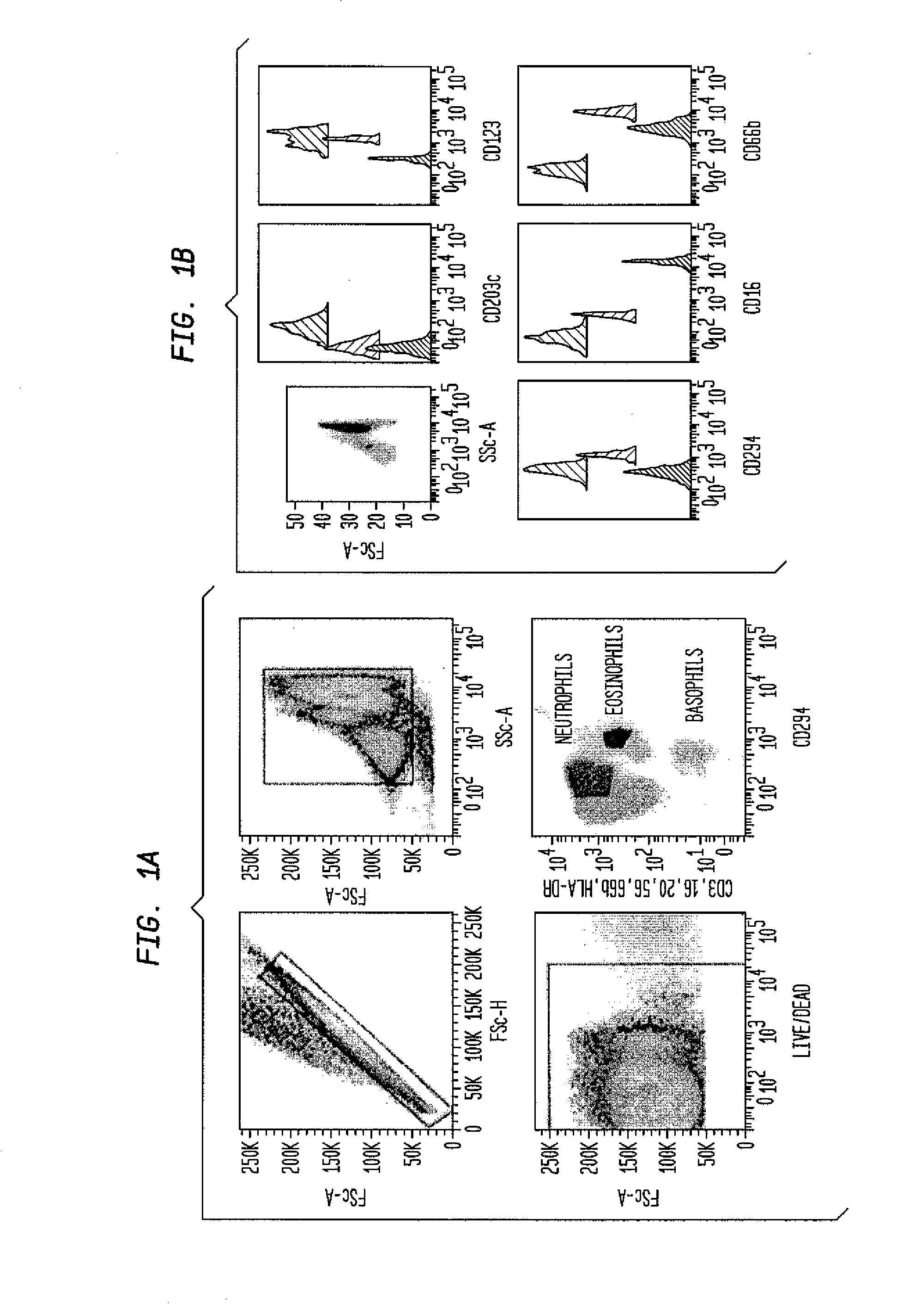
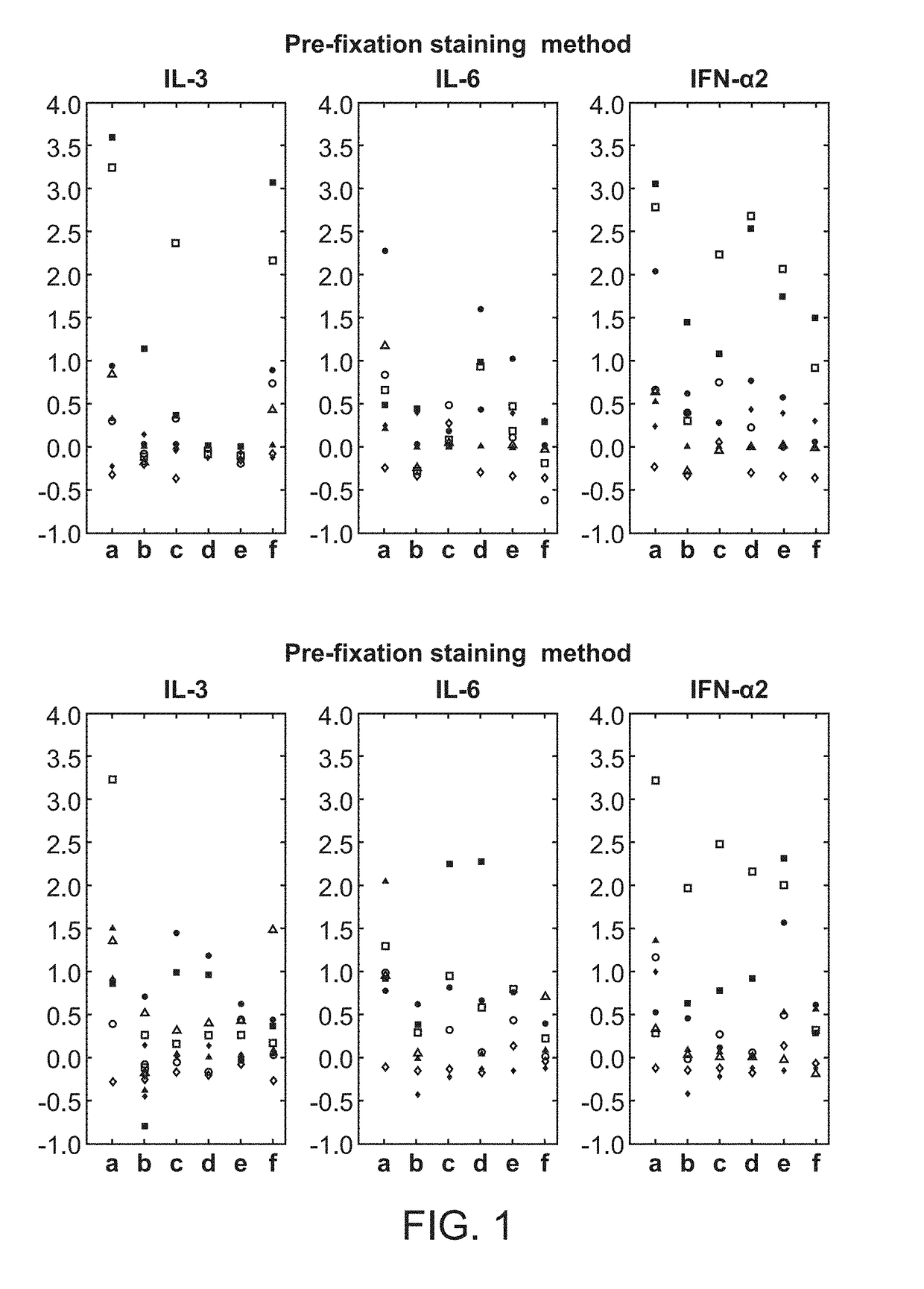
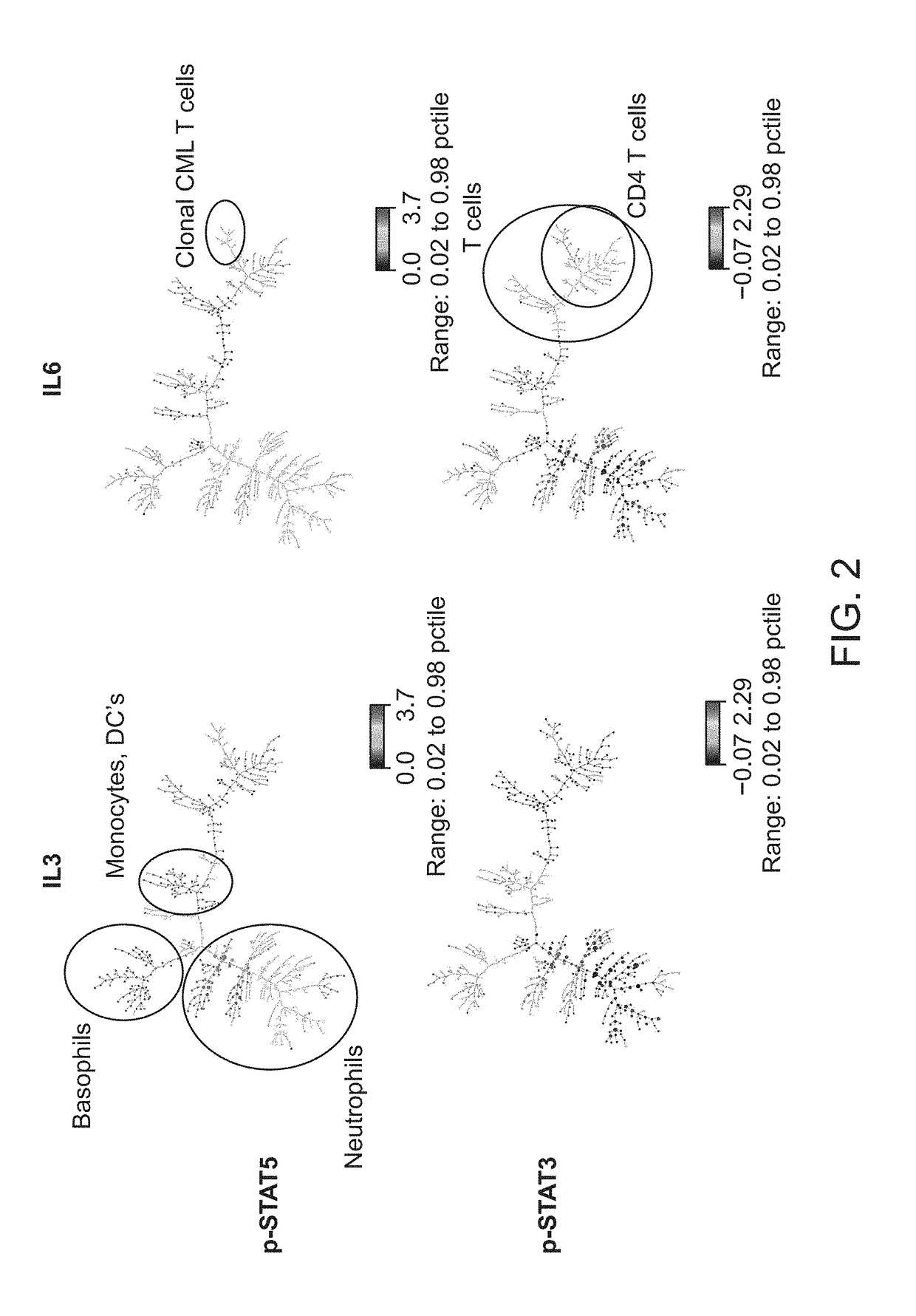
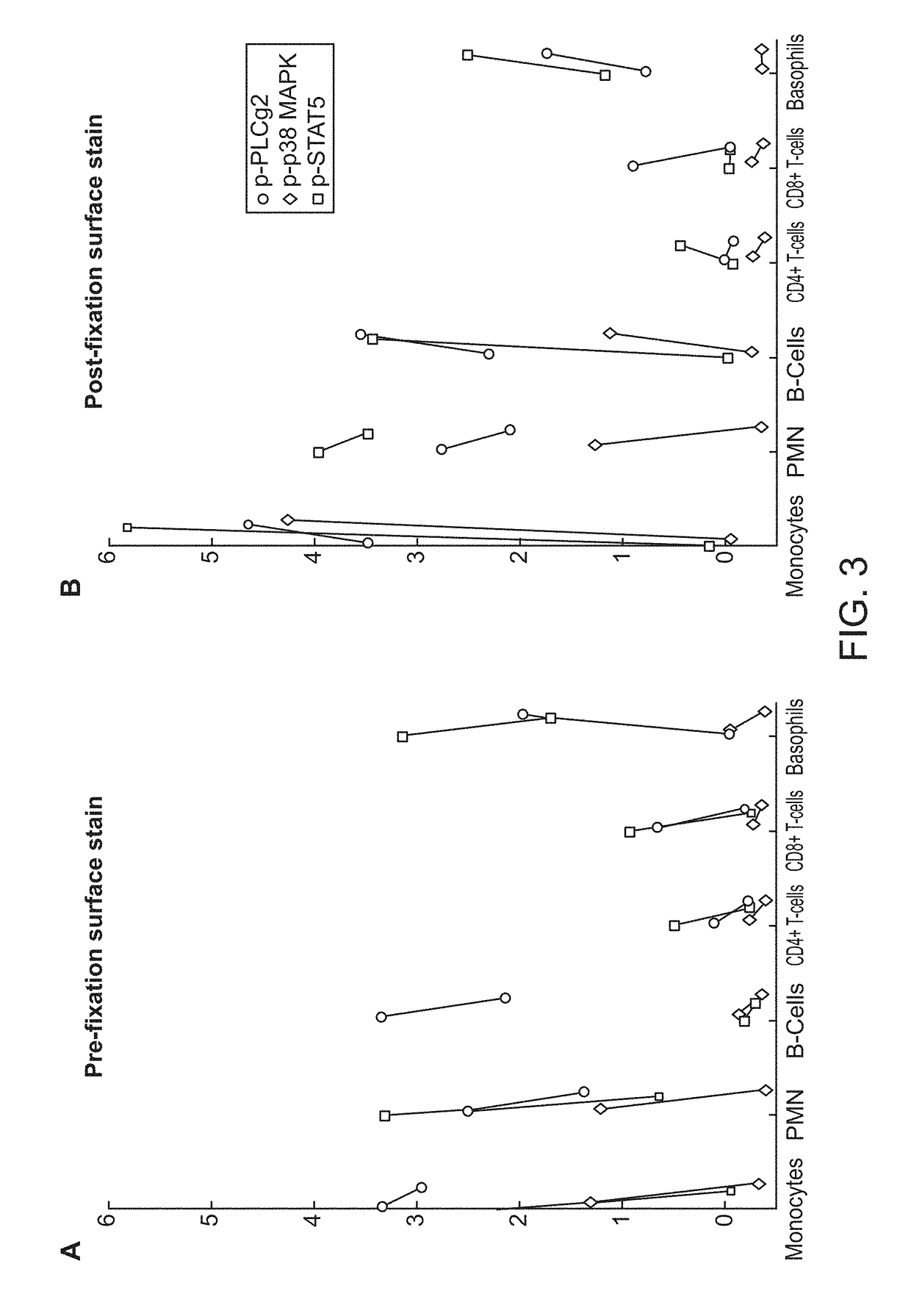
ActiveUS20180252708A1Facilitate data interpretationEasy to explainDisease diagnosisIndividual particle analysisMarker selectionProgenitor cell
The present invention recognizes that current clinical laboratory testing methods for multiparametric single cell analysis are limited to analysis of intact live cells, and are insufficient for identification of signaling activation profile defining certain cell types, including but not limited to neoplastic and immunologically activated cell subsets. One aspect of the present invention generally relates to marker selection in panels to include proteins routinely assessed in standard FCM, while preferably also incorporating markers for surface receptor proteins within activated signaling cascades. A further aspect of the present invention generally relates to panel design for the following indications in neoplastic and non-neoplastic clinical applications as examples of the technology: (a) identification of CML progenitor cell subsets in the setting of disease recurrence after treatment discontinuation or relapse due to treatment resistance, and (b) characterization of activated basophils to predict the severity of an allergic response. Another aspect of the present invention generally relates to methods to measure levels of surface and IC biomarkers in separate or combined assays for robust characterization of each or select cell compartment, and data analysis based on results from each or all method(s) used for optimal detection of the markers. A further aspect of the present invention generally relates to the identification and profiling of cell subpopulations based on analysis of surface markers including those associated with lineage and maturation of cell types and receptor proteins, and the corresponding IC phosphoproteins including those in activated signaling cascades to predict certain disease states or response to treatment.
Owner:DEEPATH MEDICAL
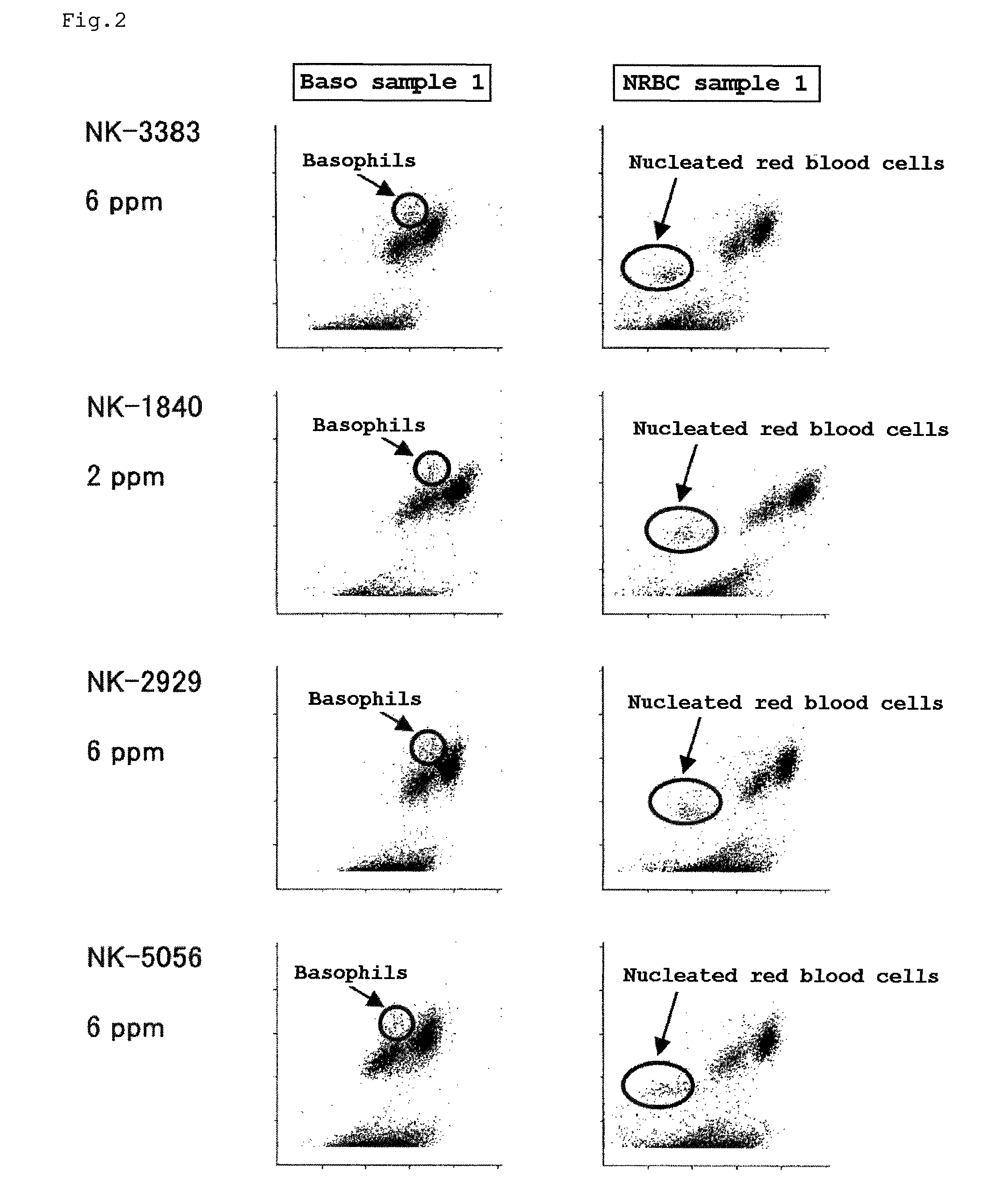
Reagent for sample analysis, reagent kit for sample analysis and method for sample analysis
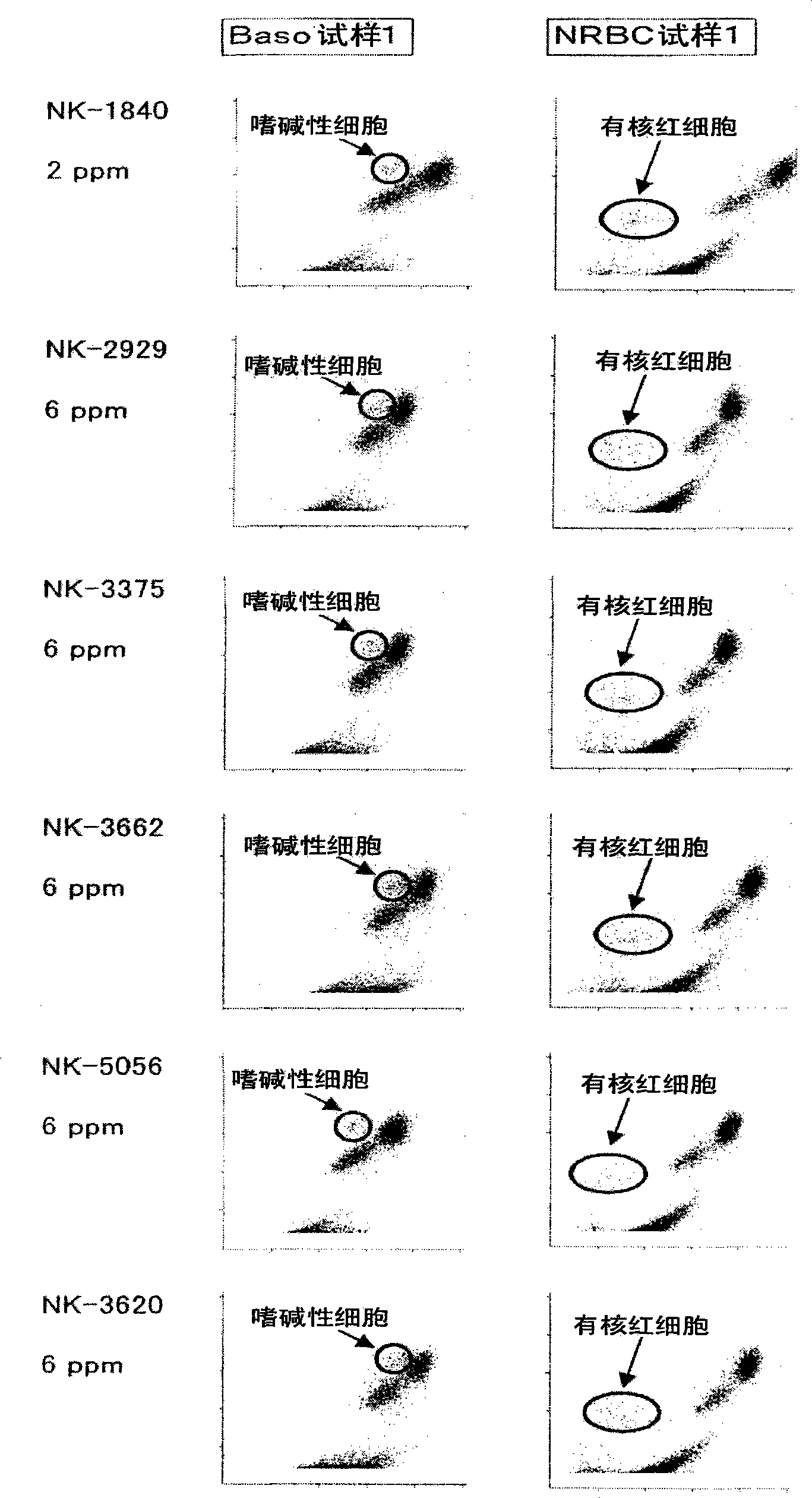
A reagent for measuring a basophil and / or a nucleated red blood cell is described. The reagent comprises (a) an alcohol having 4 to 8 total carbon atoms, and (b) one or more fluorescent dyes selected from the group consisting of a compound of the general formula (I) and a compound of the general formula (II).
Owner:SYSMEX CORP
White blood cell classification reagent and method
InactiveCN110031383AImprove accuracyImprove reliabilityIndividual particle analysisDiseaseBasophil cell
The invention relates to the technical field of blood analysis, in particular to a white blood cell classification reagent and method. The white blood cell classification reagent is prepared from thecomponents in parts by weight: 0.1-5 parts of a zwitterionic surfactant, 0.5-10 parts of a nonionic surfactant, 0.1-30 parts of a cosolvent, 5-30 parts of an osmotic pressure regulator, 0.1-2 parts ofa preservative, 5-20 parts of a buffer agent, 0.001-0.2 part of dye and 0.1-2 parts of a fixing agent. The white blood cell classification reagent can carry out quartered grouping (4 groups corresponding to neutrophils and basophils, lymphocytes, monocytes and eosinophils) on normal blood samples and can further carry out detection on blood samples containing juvenile white blood cells of patients with specific diseases, and the blood samples of the patients can be divided into 5 groups in total which are the 4 groups corresponding to neutrophils and basophils, lymphocytes, monocytes and eosinophils and a group corresponding to the juvenile white blood cells.
Owner:SONOSCAPE MEDICAL CORP
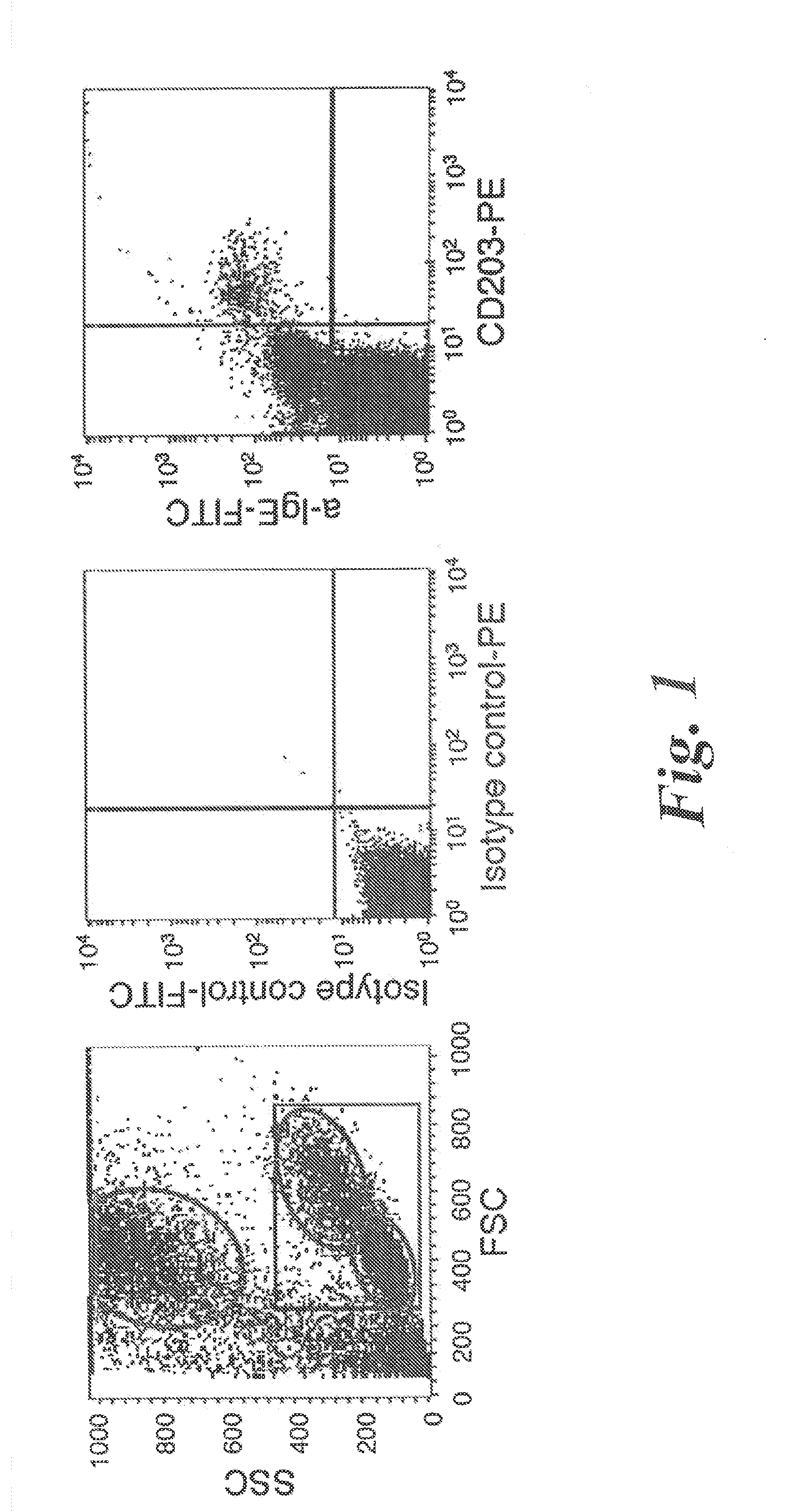
Method for detecting allergies
InactiveUS20050003461A1Successfully diagnoseBiological material analysisBiological testingMast cellMicroorganism
The present invention relates to a method for detecting allergies, with a blood sample initially being incubated with a cell-influencing agent, preferably interleukin IL-3, and then being incubated with an allergen, and with this blood sample subsequently being incubated with an antibody which binds to the surface structures on basophils and / or mast cells and / or precursor cells of basophils and / or mast cells to which the antibody 97A6 can bind, which antibody is produced and released by hybridoma cells which were deposited in the Deutsche Sammlung von Mikroorganismen und Zellkulturen [German collection of microorganisms and cell cultures] GmbH, DSMZ, under number DSM ACC 2297, in accordance with the Budapest Treaty, on Dec. 2, 1997, and with the antibodies which have bound to the basophils and / or mast cells and / or precursor cells of the basophils and / or mast cells then being quantified.
Owner:UNIV TUBINGEN
Composition containing extract or fraction of genus justicia plant
ActiveUS20170360862A1Prevent, treat or improve allergic diseaseCosmetic preparationsSenses disorderAntibody secretionMast cell
The present invention relates to a pharmaceutical composition, food composition and cosmetic composition for preventing, treating, or improving allergic diseases comprising extract or fraction of a plant of the Justicia genus as an active ingredient. The extract or a fraction of a plant of the Justicia genus according to the present invention can inhibit IgE antibody secretion and the degranulation of mast cells and basophils, and exhibits an excellent anti-allergic effect, and thus can effectively prevent, treat, or improve allergic diseases.
Owner:DONG WHA PHARM CO LTD
A five-classification method for leukocytes based on deep learning
ActiveCN106248559BAvoid Classification ErrorsImprove effectivenessIndividual particle analysisImaging processingWhite blood cell
The invention belongs to the field of medical image processing, and relates to a five-classification technology for leukocytes in a human peripheral blood cell image, particularly to a leukocyte five-classification method based on deep learning. According to the leukocyte five-classification method, leukocytes are detected from a microscope image by using simple color-component relationships and morphological operations, basophils and eosinophils are identified by using particle characteristics and SVM, the remaining cell image characteristics are automatically extracted by using a convolutional neural network, and finally the remaining three-classification is achieved by using random forest. With the leukocyte five-classification method of the present invention, the errors caused by segmentation in the traditional method can be avoided, the leukocyte five-classification problem can be effectively solved, and the cells in different databases can achieve the good classification results.
Owner:CHINA JILIANG UNIV +1
Administering IgE antagonists during pregnancy to ameliorate allergic diseases in the offspring
InactiveUS20020006402A1Less immunogenicLow immunogenicityAntibody ingredientsImmunoglobulinsAllergyIge binding
The invention relates to IgE antagonists, including monoclonal antibodies, and their use in ameliorating asthma and allergic diseases in offspring of mothers treated during pregnancy with such antagonists. The preferred IgE antagonists do not induce release of the mediators of allergy. One example of such IgE antagonists are anti-IgE antibodies which bind to secreted IgE, to membrane IgE on the surface of IgE-producing B cells, but not to IgE bound to the Fc∈RI on the surface of basophils or mast cells. Preferably, these antibodies also do not bind to IgE bound to Fc∈RII receptors. It is also preferable if these antibodies have human IgG1 or IgG3 constant regions, as well as further human portions, if desired.
Owner:TANOX
Cosmetic composition capable of balancing and repairing microbial barrier of skin
InactiveCN109464324AInhibition releaseBlock allergic reactionsCosmetic preparationsToilet preparationsCholesterolAlcohol sugars
The invention discloses a cosmetic composition capable of balancing and repairing a microbial barrier of skin. The cosmetic composition comprises the following traditional Chinese medicine extract rawmaterials in percentage by weight: 5% to 15% of dried tangerine or orange peel, 5% to 15% of calendula, 5% to 15% of witch hazel, 5% to 15% of chamomile, 5% to 15% of Astragalus membranaceus, 5% to 15% of white poria, 5% to 15% of Typhonium giganteum, 5% to 15% of cortex dictamni, 5% to 15% of ginseng, 0.5% to 1% of xanthan gum, 1% to 2% of vitamin E, 1% to 2% of glycerinum, 0.5% to 1% of a thickening agent, 1% to 2% of magnetized water, calendula oil, a witch hazel extracting solution, apricot sugar alcohol, a chamomile extracting solution and the like. Moreover, through antioxidant ingredients capable of efficiently eliminating free radicals, mast cells and basophil films are protected from being affected by the free radicals, so that the release of sensitization factors is inhibited, the occurrence of an anaphylactic reaction is blocked fundamentally, and physiologic lipids can be actively supplemented for the skin for the repair of the barrier through ceramide, cholesterol and free fatty acid.
Owner:佛山市奥姿美生物科技有限公司
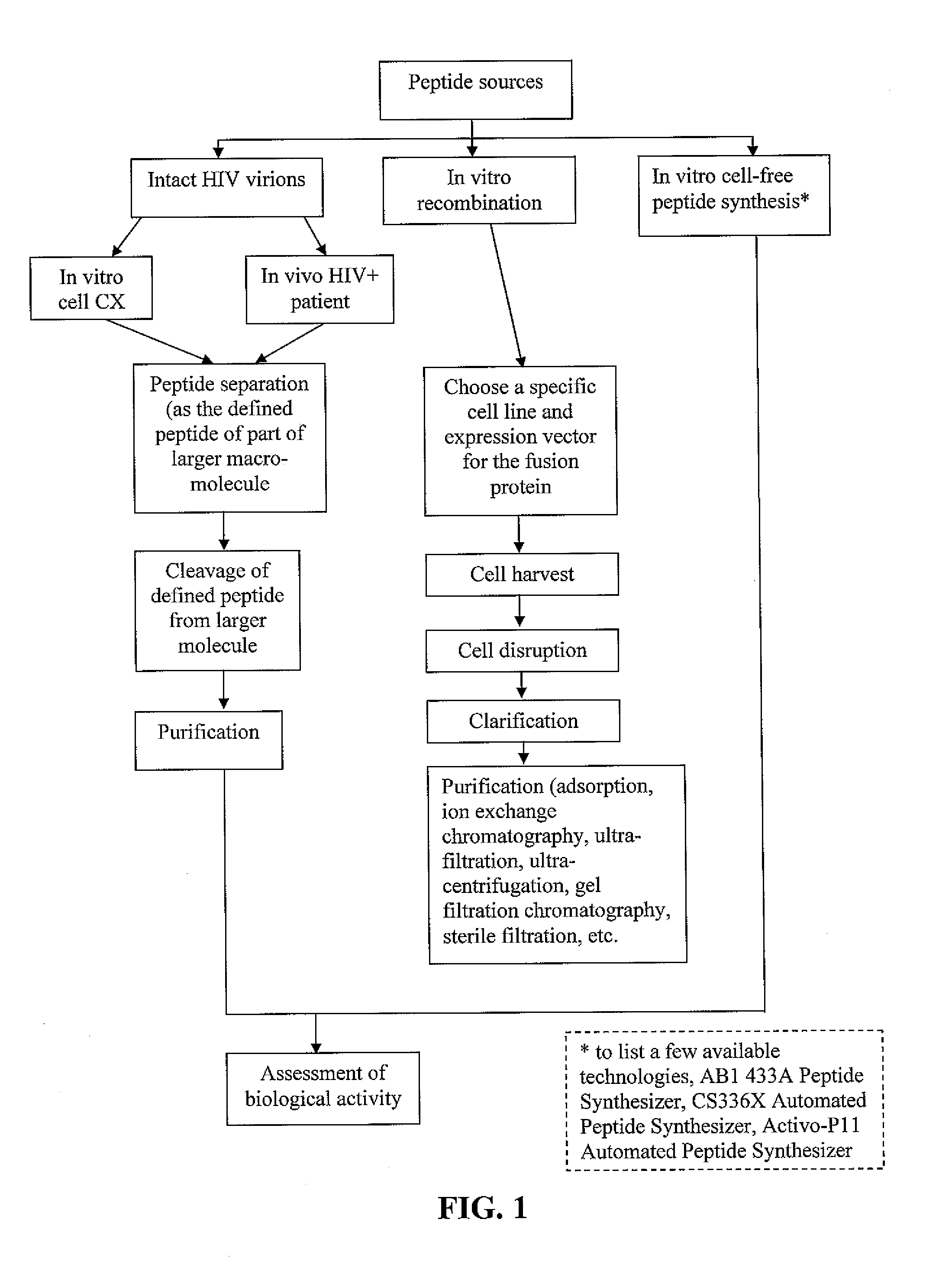
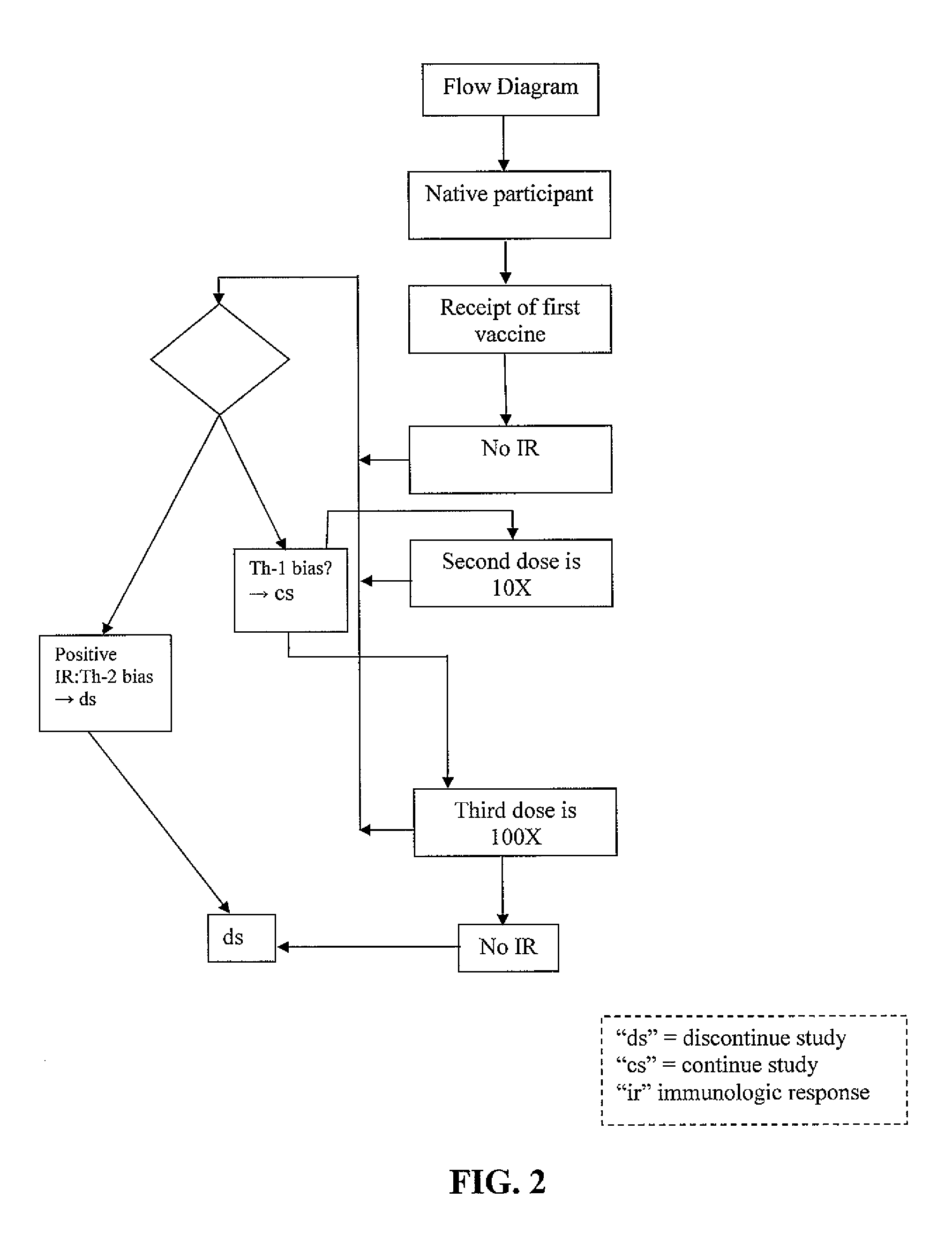
Methods of inducing TH-1 immune responses to HIV-1 by administering UV/psoralen-treated desialated inactiviated HIV-1 virions deficient in CD55 and CD59
Administration protocols for a fusion protein, matrix protein and psoralen inactivated HIV based immunogenic composition that induces an immune response to HIV. The immunogenic compositions are based on HIV biologically active fusion peptide, matrix peptide, or psoralen inactivated HIV. The number of doses is 3X. The starting dose for an adult is 1x109-1x1010. The starting dose for an adolescent is ½(1x109-1x1010). The starting dose for a pediatric patient is ¼(1x109-1x1010). The second dose will consist of 1 / 10th of starting concentrations. The third dose will consist of 1 / 100th of starting concentrations. This will facilitate a Th-1 response. The days of administration are days 1; 30; and 180. Alternatively the days of administration are days 1; 20-40; and 160-200. The site of administration is one that targets lymphatic tissue. Adjuvant is administered before, simultaneous with or after each dose of the immunogenic compositions. Adjuvants are used to promote a Th-1 immune response and include a leukotriene receptor antagonist such as Montelukast, a mast cell and basophil stabilizer such as Cromolyn, and a prostaglandin synthetase inhibitor such as Indomethacin. Th-1 immune responses to the immunogenic compositions are monitored. The 3X cycle will repeat on until a Th-1 immune response is observed. At that point, the immunogenic composition administered could then decline by a factor of 10 for two more vaccination procedures.
Owner:KARP NELSON M
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com