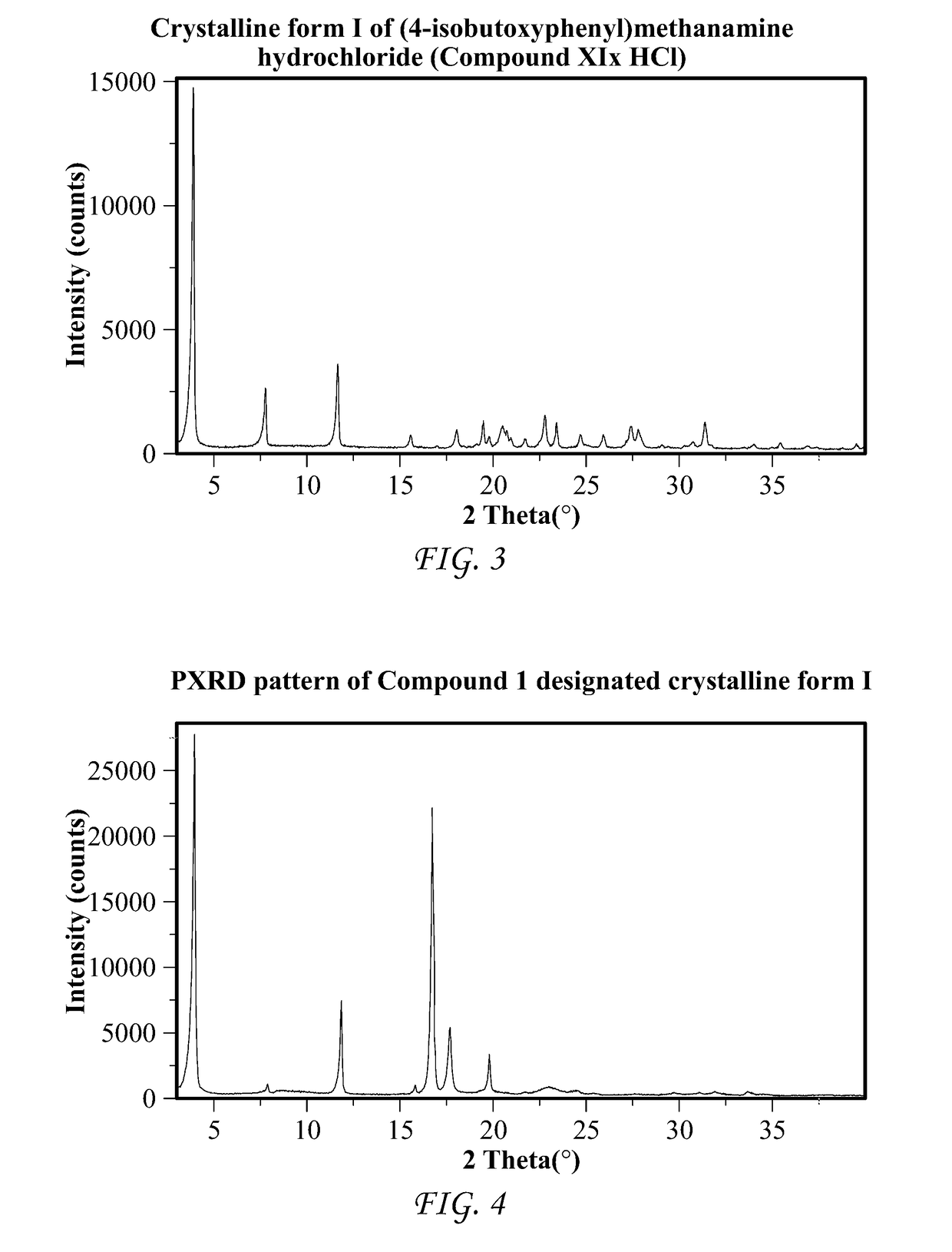
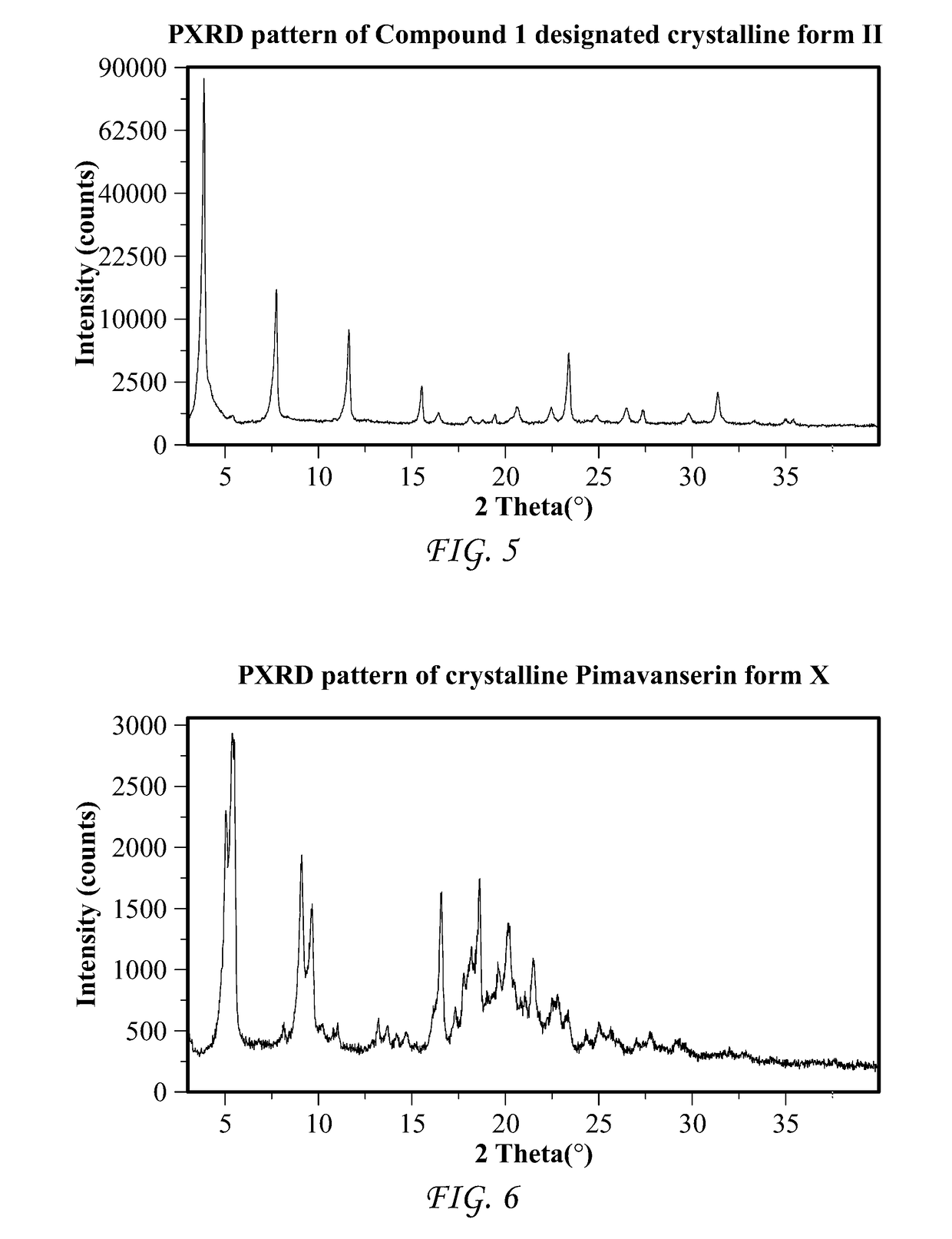
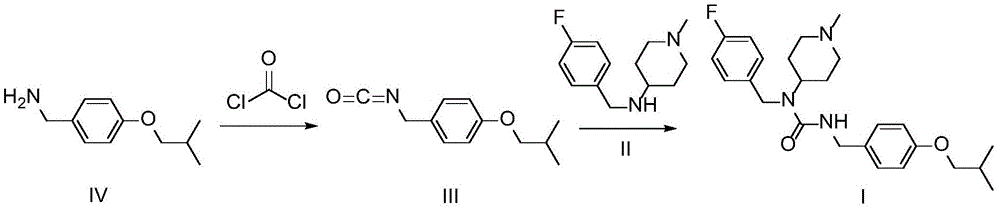
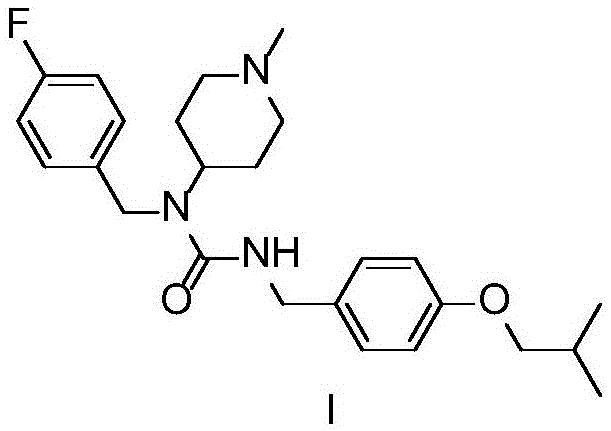
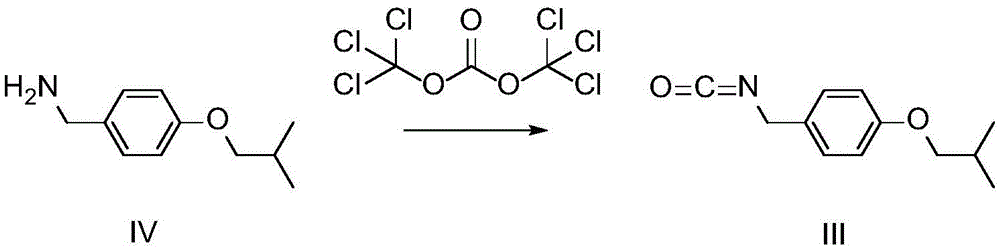
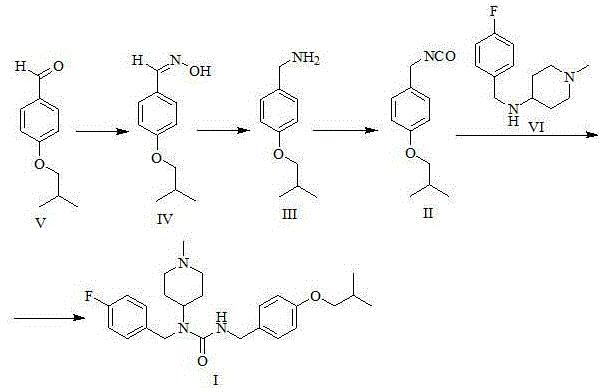
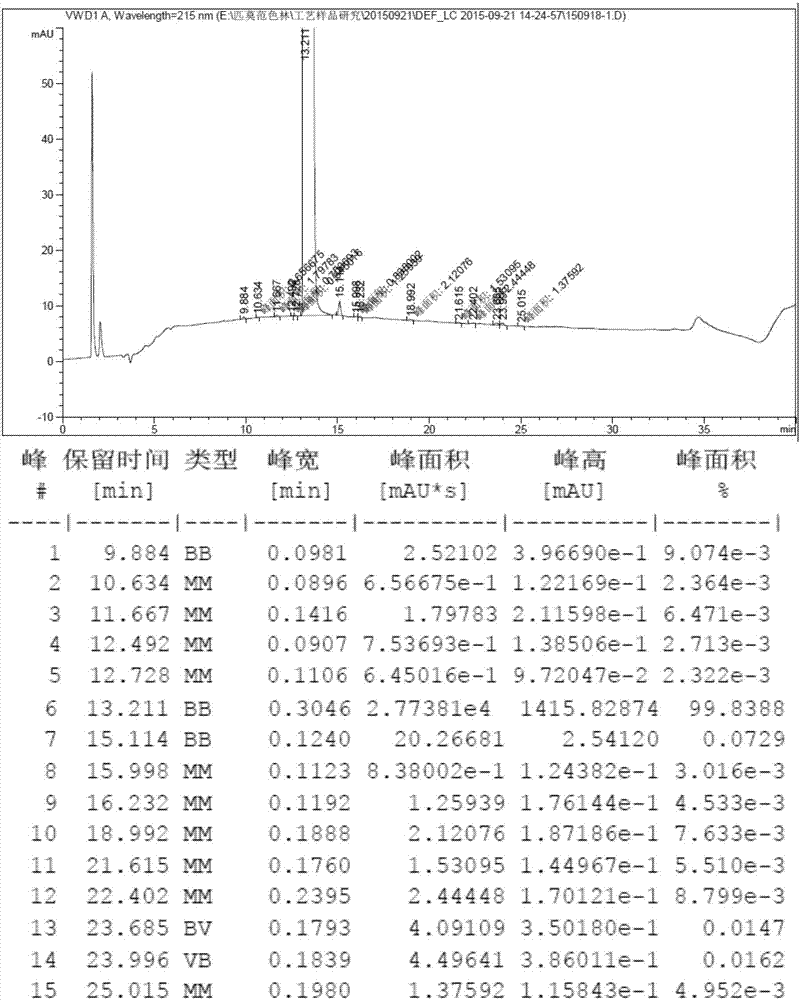
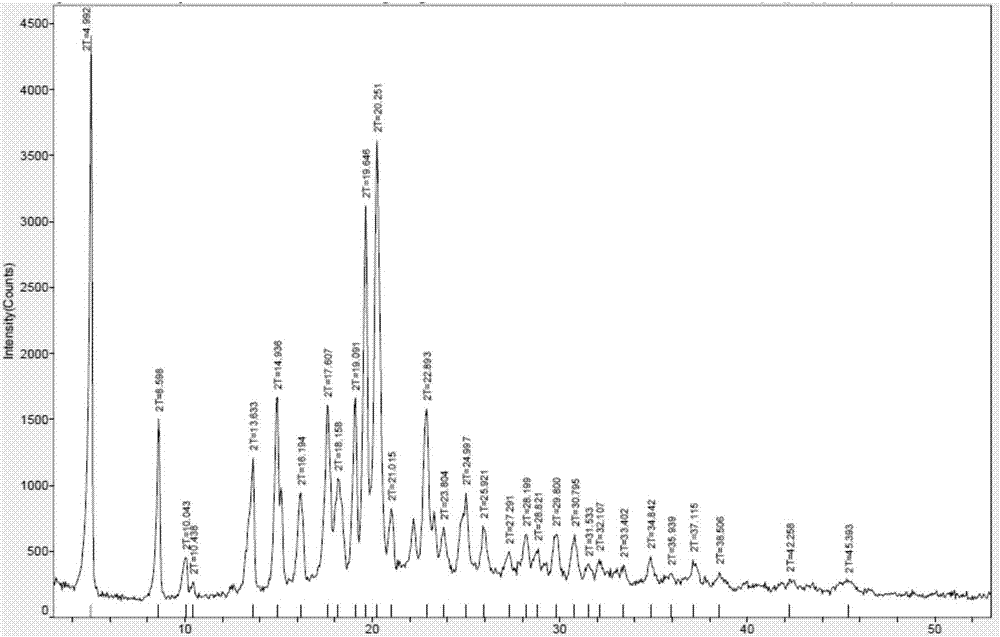
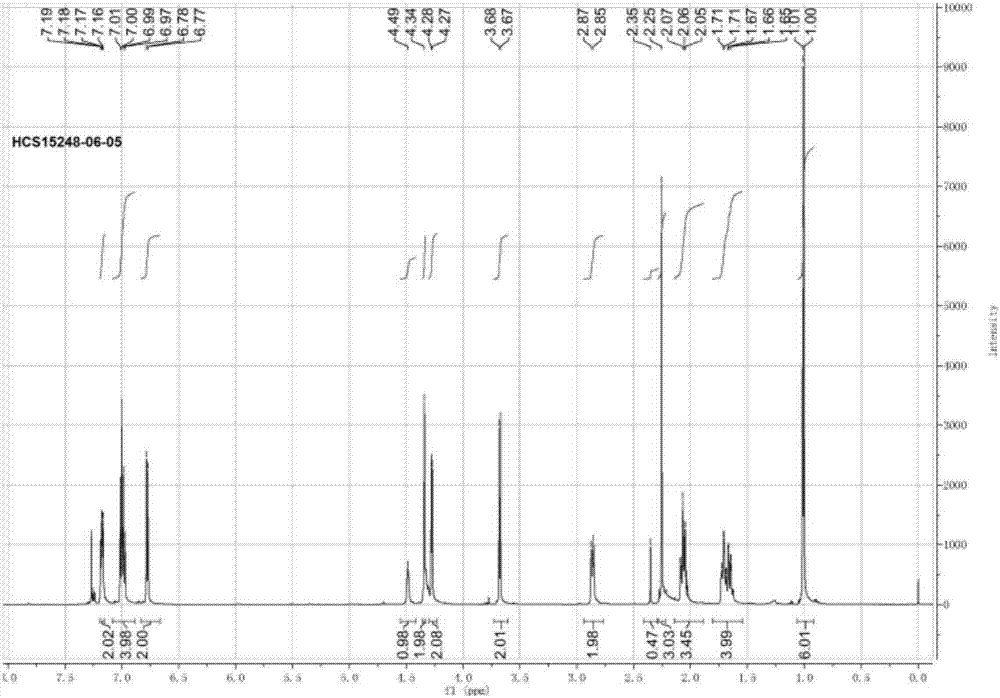
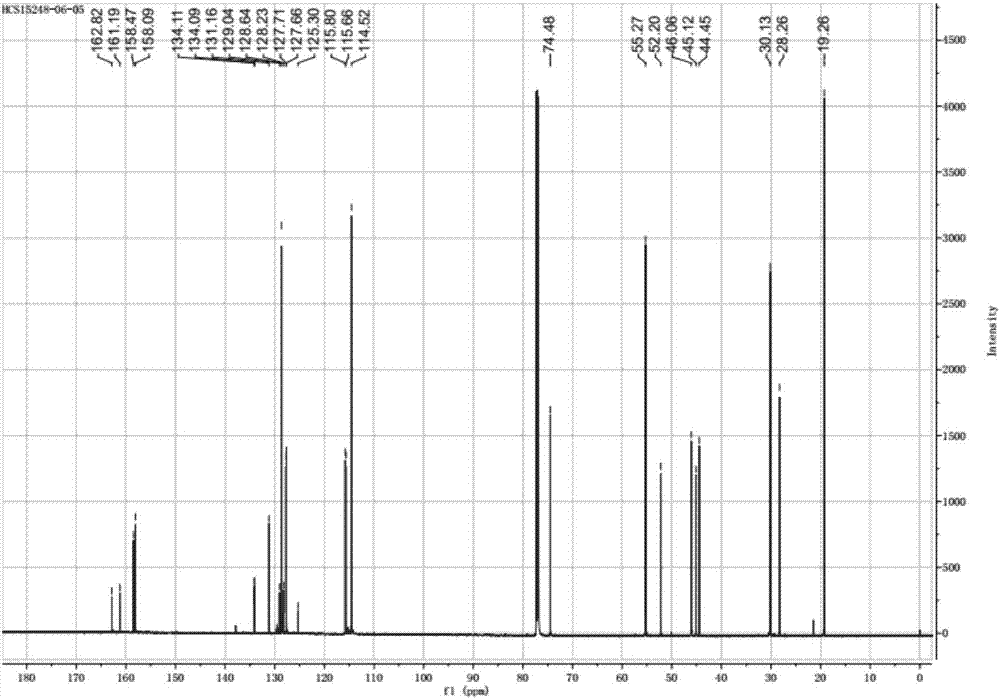
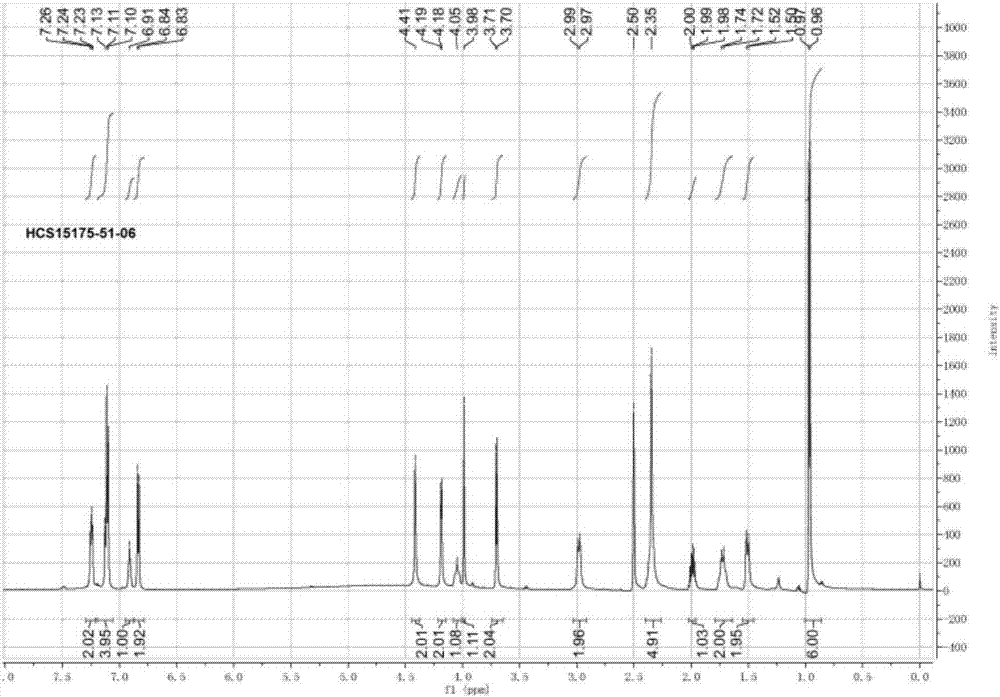
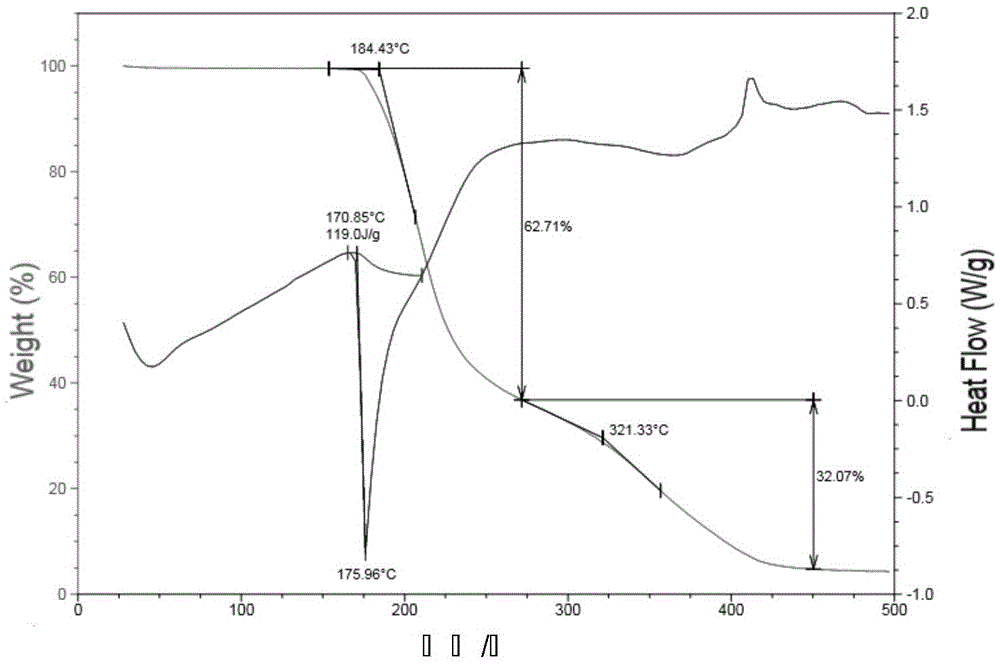
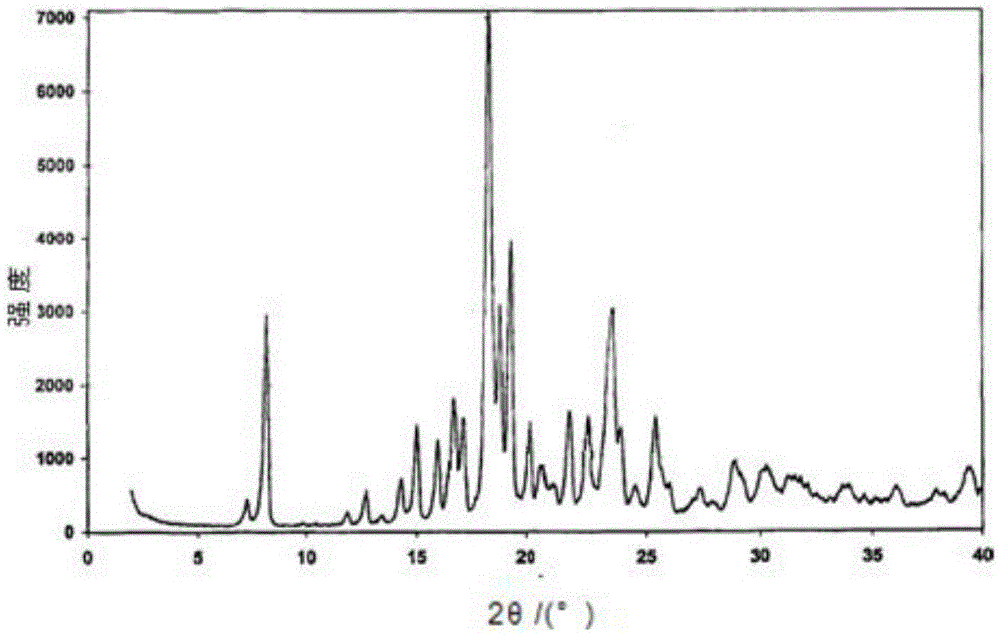
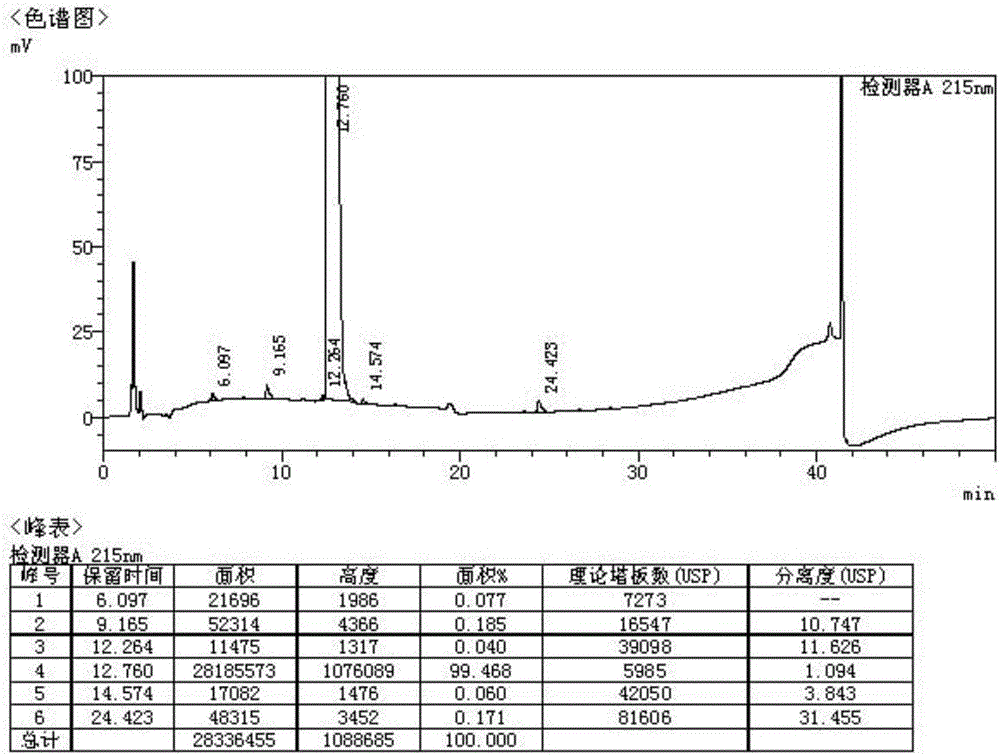
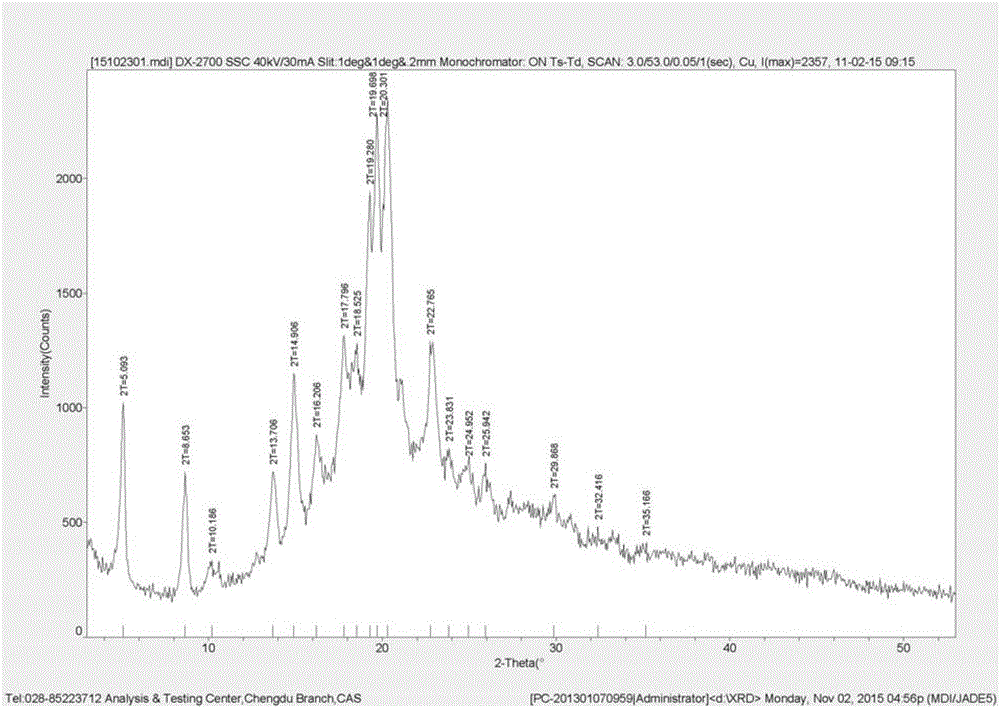
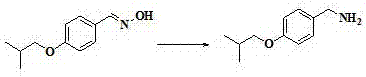Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Pimavanserin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pimavanserin is used to treat the symptoms of a certain mental/mood disorder (psychosis) that might occur with Parkinson's disease.
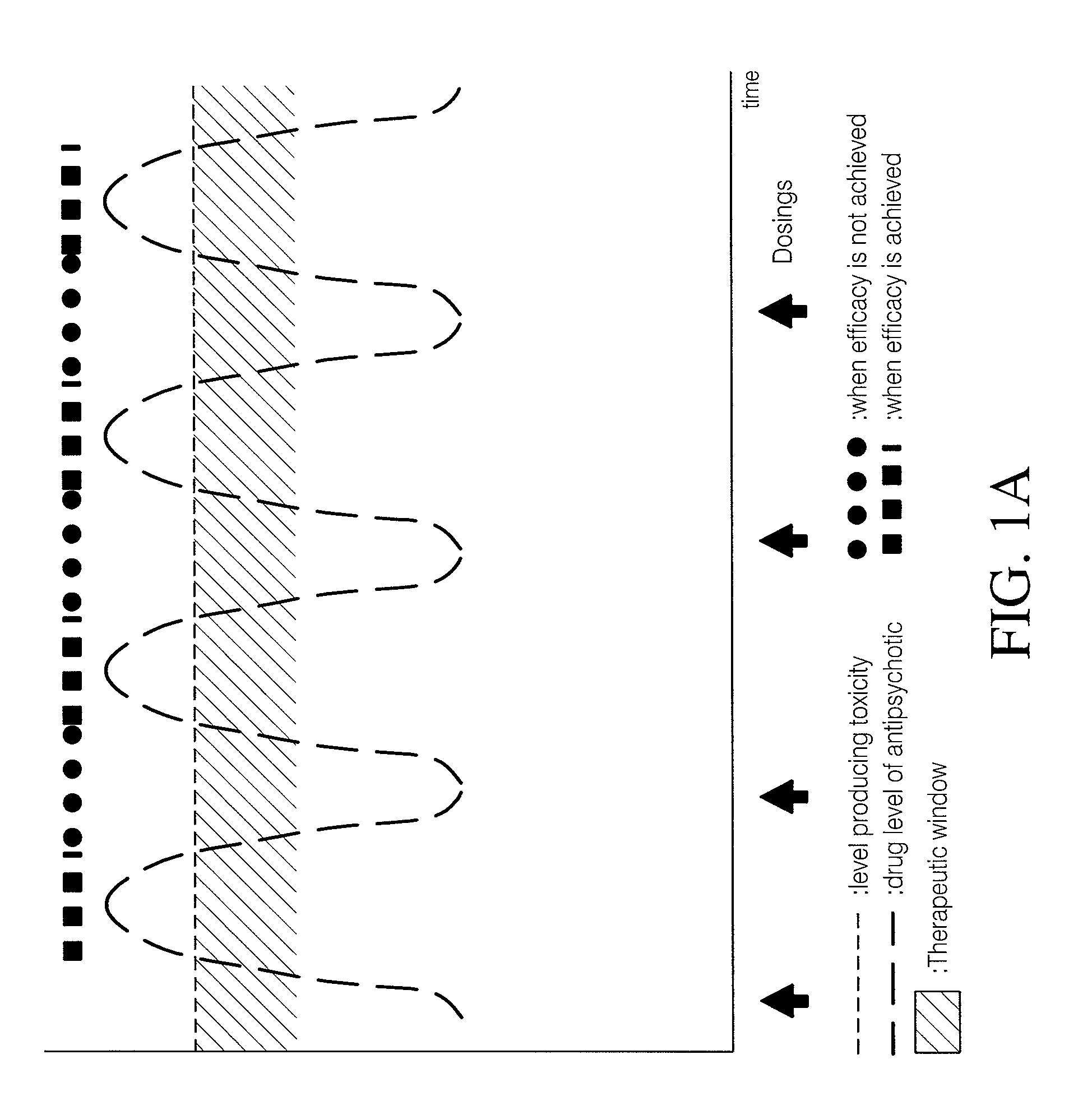
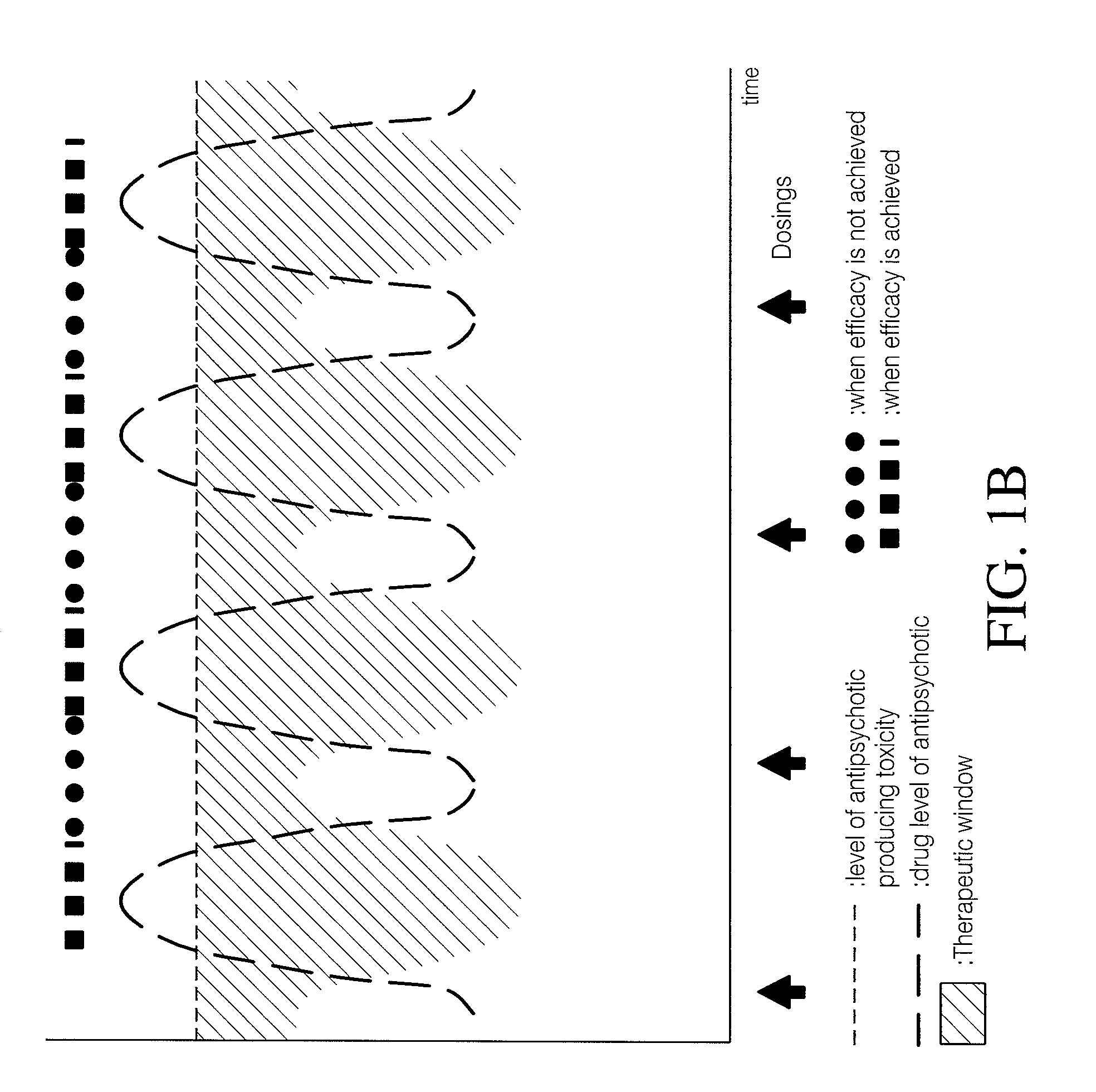
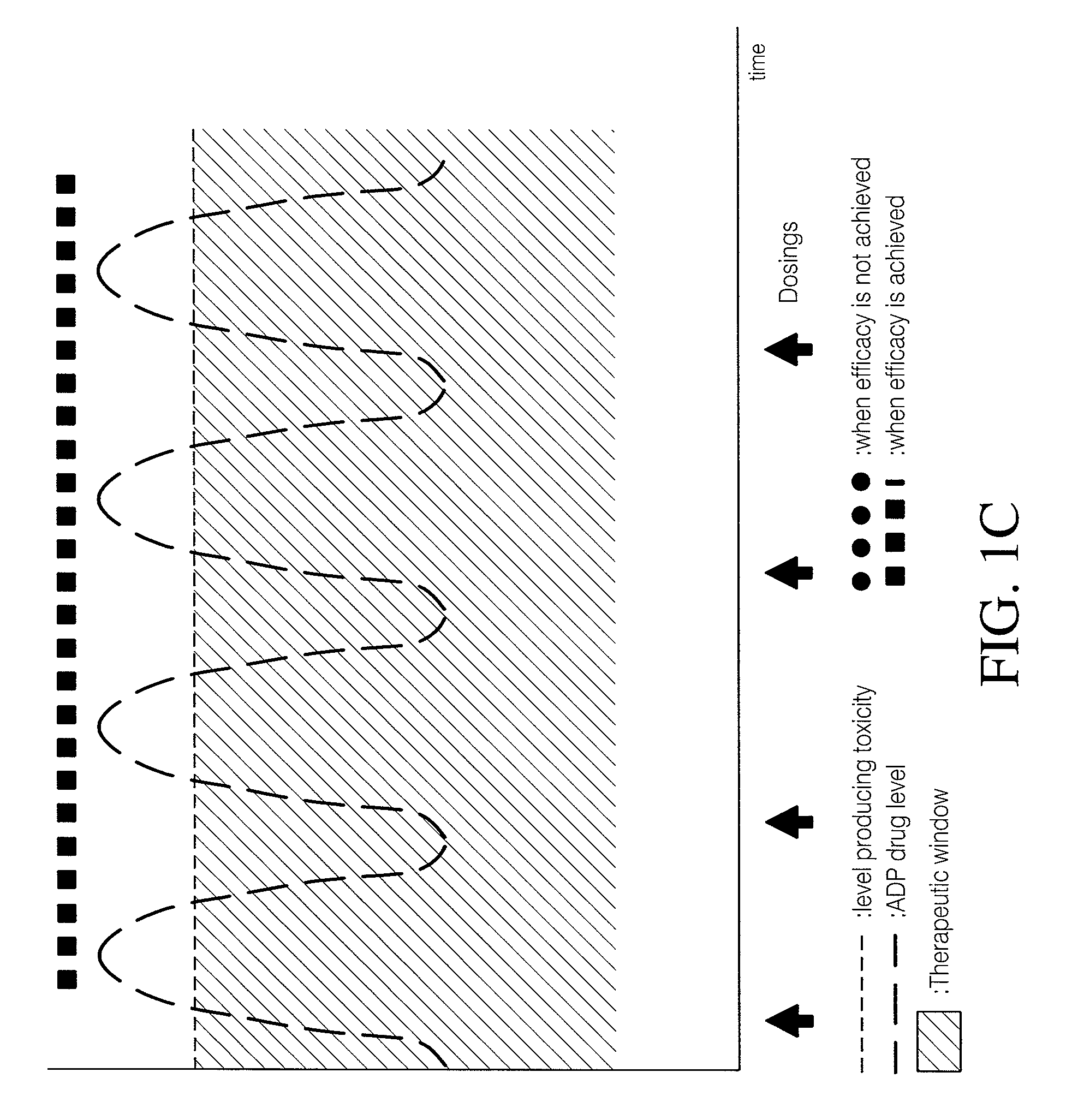
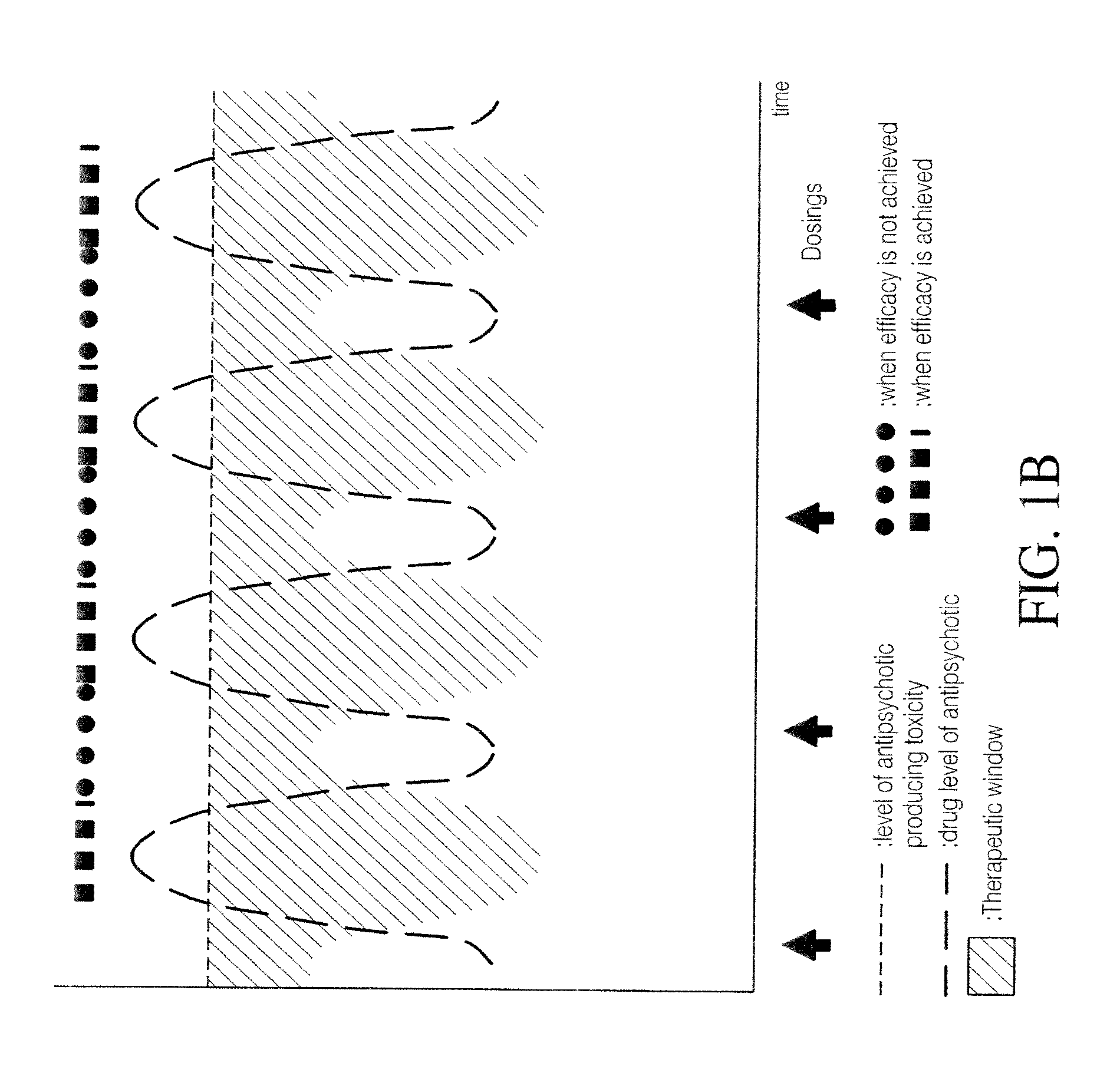
Combinations of 5-ht2a inverse agonists and antagonists with antipsychotics
InactiveUS20090053329A1Achieve effectQuick effectCompounds screening/testingBiocideSide effectAntipsychotic drug therapy
Combinations of 5-HT2A inverse agonists or antagonists such as pimavanserin with antipsychotics such as risperidone are shown to induce a rapid onset of antipsychotic action and increase the number of responders when compared to therapy with the antipsychotic alone. These effects can be achieved at a low dose of the antipsychotic, thereby reducing the incidence of side effects. The combinations are also effective at decreases the incidence of weight gain and increased glucose or prolactin levels caused by the antipsychotic.
Owner:ACADIA PHARMA INC
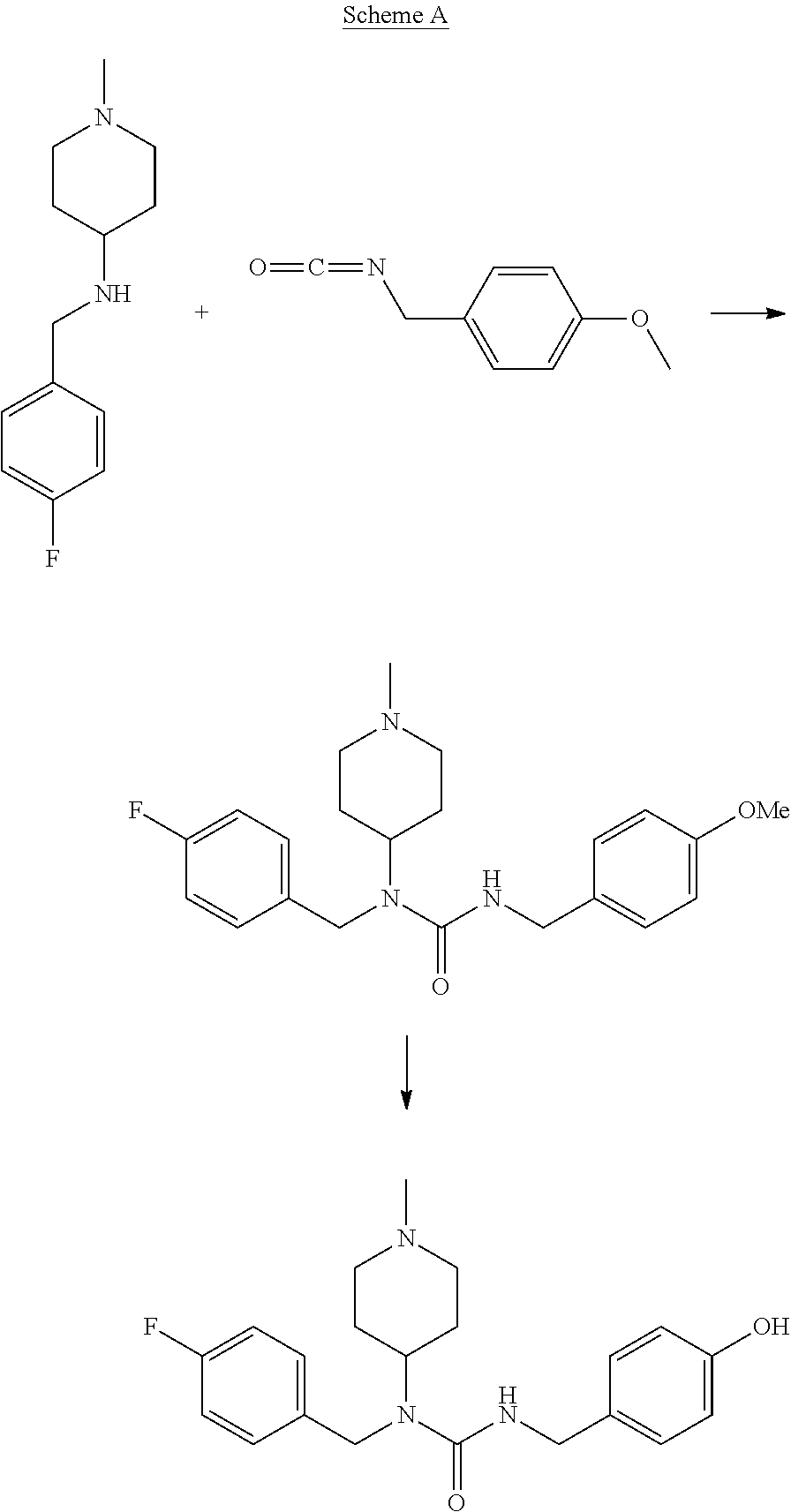
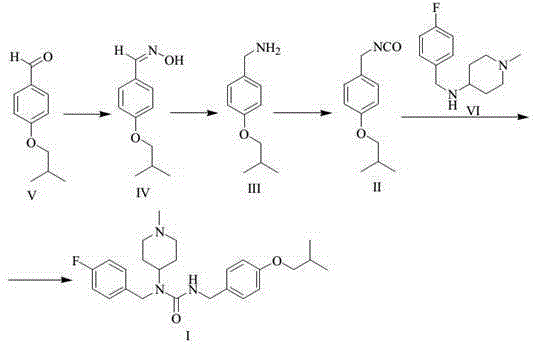
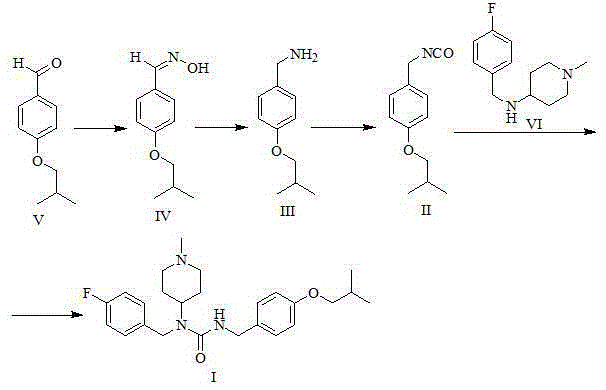
Intermediate of pimavanserin and similar compound thereof, and preparation method thereof, and method for preparing pimavanserin and similar compound thereof
ActiveCN105418460ALow priceEasy to separate and purifyCarbamic acid derivatives preparationOrganic compound preparationChemical compoundPhosgene
The present invention relates to an intermediate of pimavanserin and a similar compound thereof, and a preparation method thereof, and a method for preparing pimavanserin and a similar compound thereof. According to the present invention, the raw materials required by the intermediate have characteristics of low price, easy obtaining, easy separation purification and no requirement of post-treatment, the next step reaction can be directly performed through the one-pot method to prepare the pimavanserin and the similar compound thereof, the operation is simple, and the economical, efficient, safe and environmentally friendly synthesis process is provided for the preparation of the pimavanserin and the similar compound thereof; and with the application of the intermediate to prepare the pimavanserin, the high toxicity and the use of the difficultly-operated phosgene can be avoided, and the total yield of the reaction can achieves the level in the prior art even the higher level.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Preparation method of pimavanserin
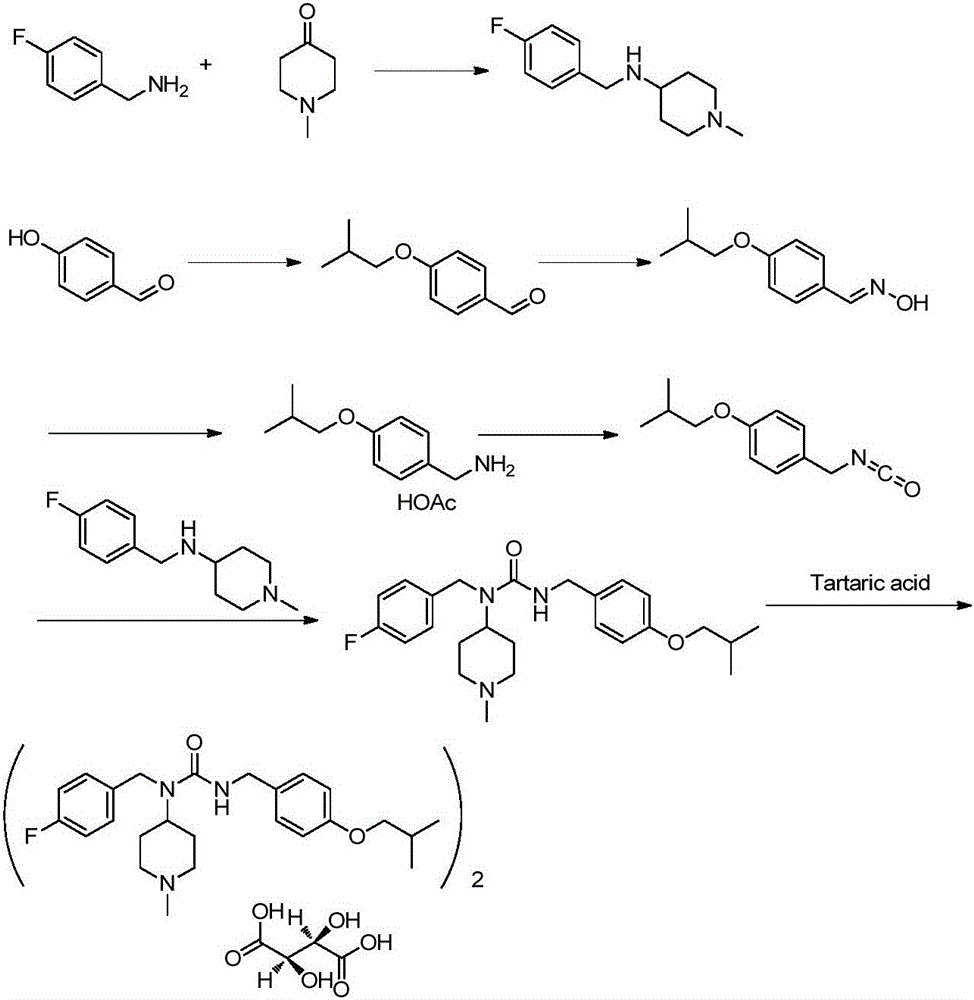
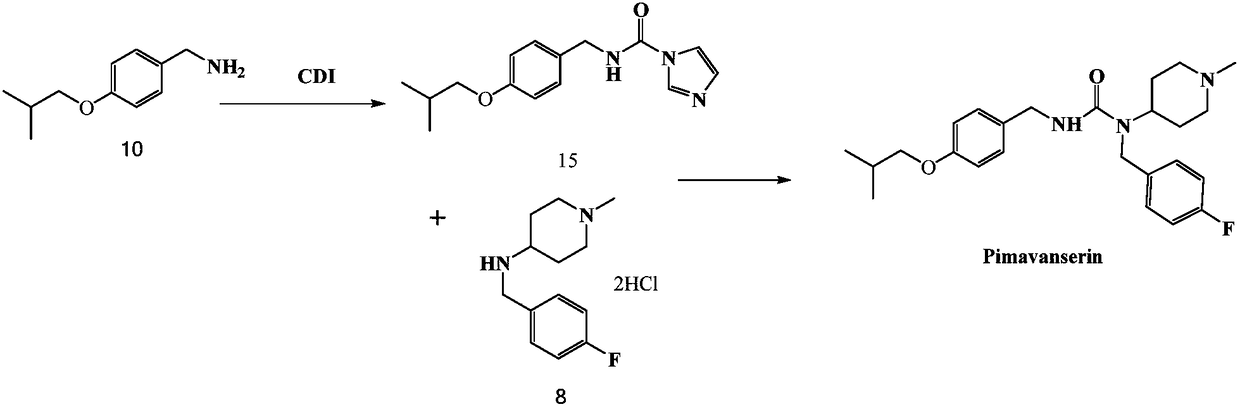
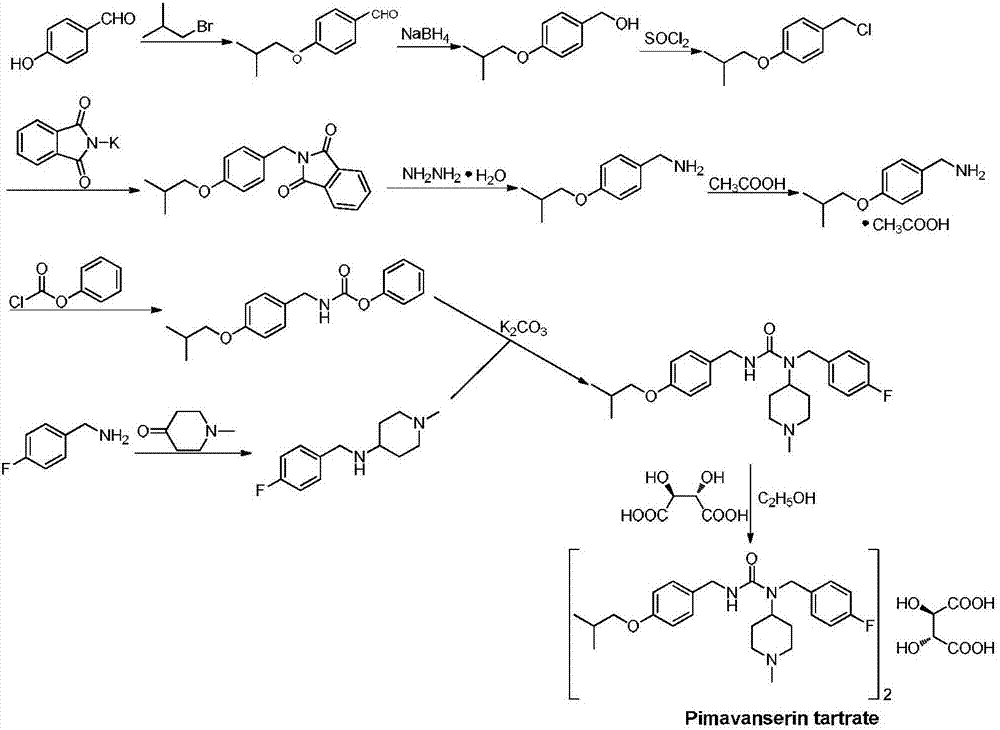
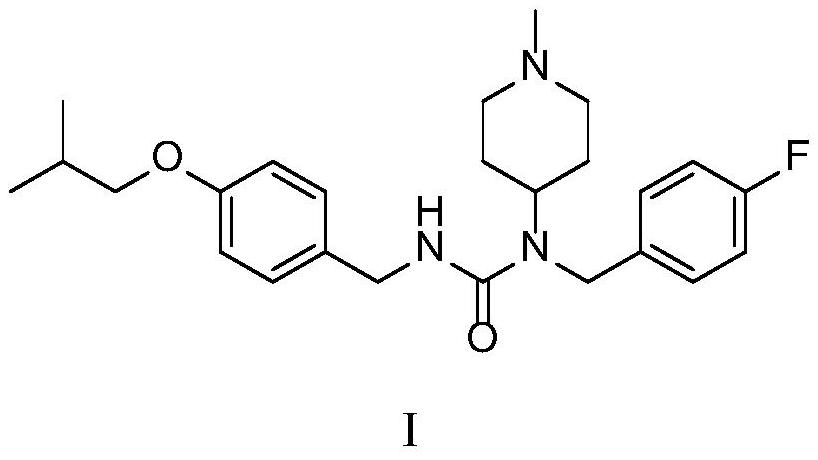
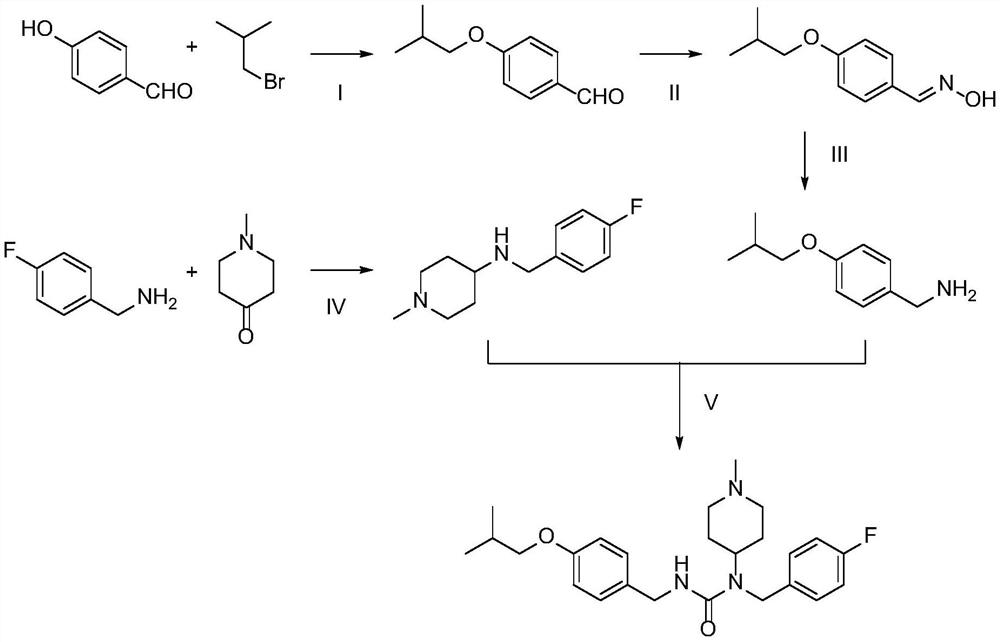
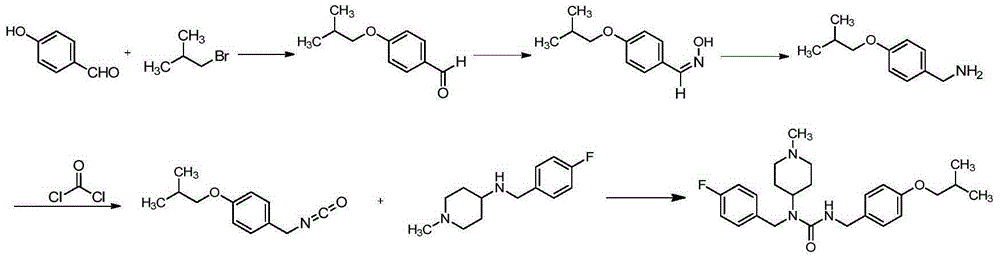
The invention discloses a preparation method of pimavanserin. The method comprises the following two steps: firstly, 4-isobutoxy benzene methylamine and carbonyl diimidazole are subjected to acylation reaction to obtain N-(4-isobutoxy phenyl)-1H-imidazole-1-formamide, and the N-(4-isobutoxy phenyl)-1H-imidazole-1-formamide and N-(4-fluorophenyl)-1-methylpiperidine-4-amine are subjected to urea reaction, so as to obtain the pimavanserin. The prepared pimavanserin is good in quality and high in yield, the reagent toxicity is relatively low, the operation is simple and easy to control, and the pimavanserin is suitable for industrial production.
Owner:NKD PHARMA CO LTD
Co-administration of pimavanserin with other agents
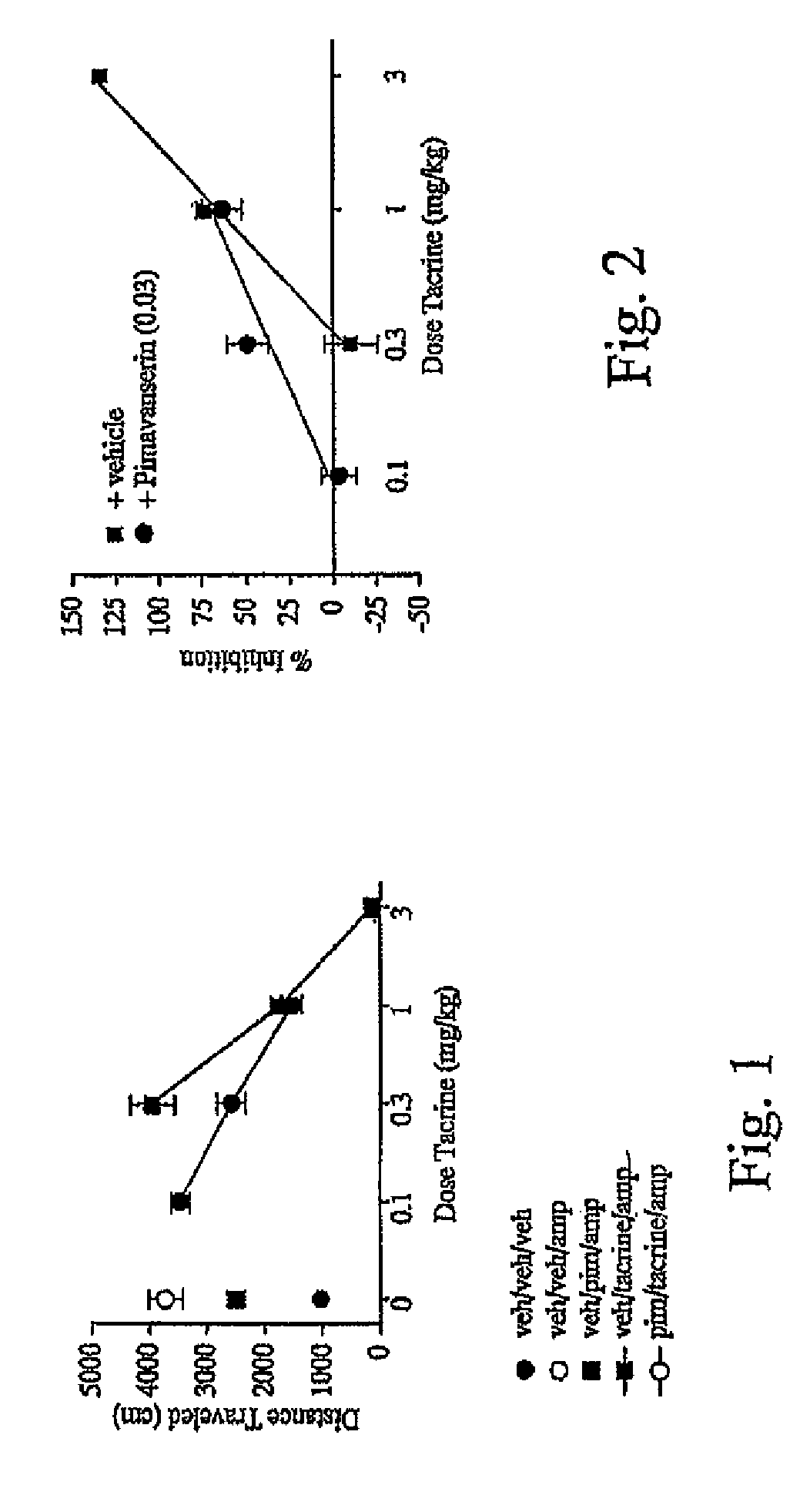
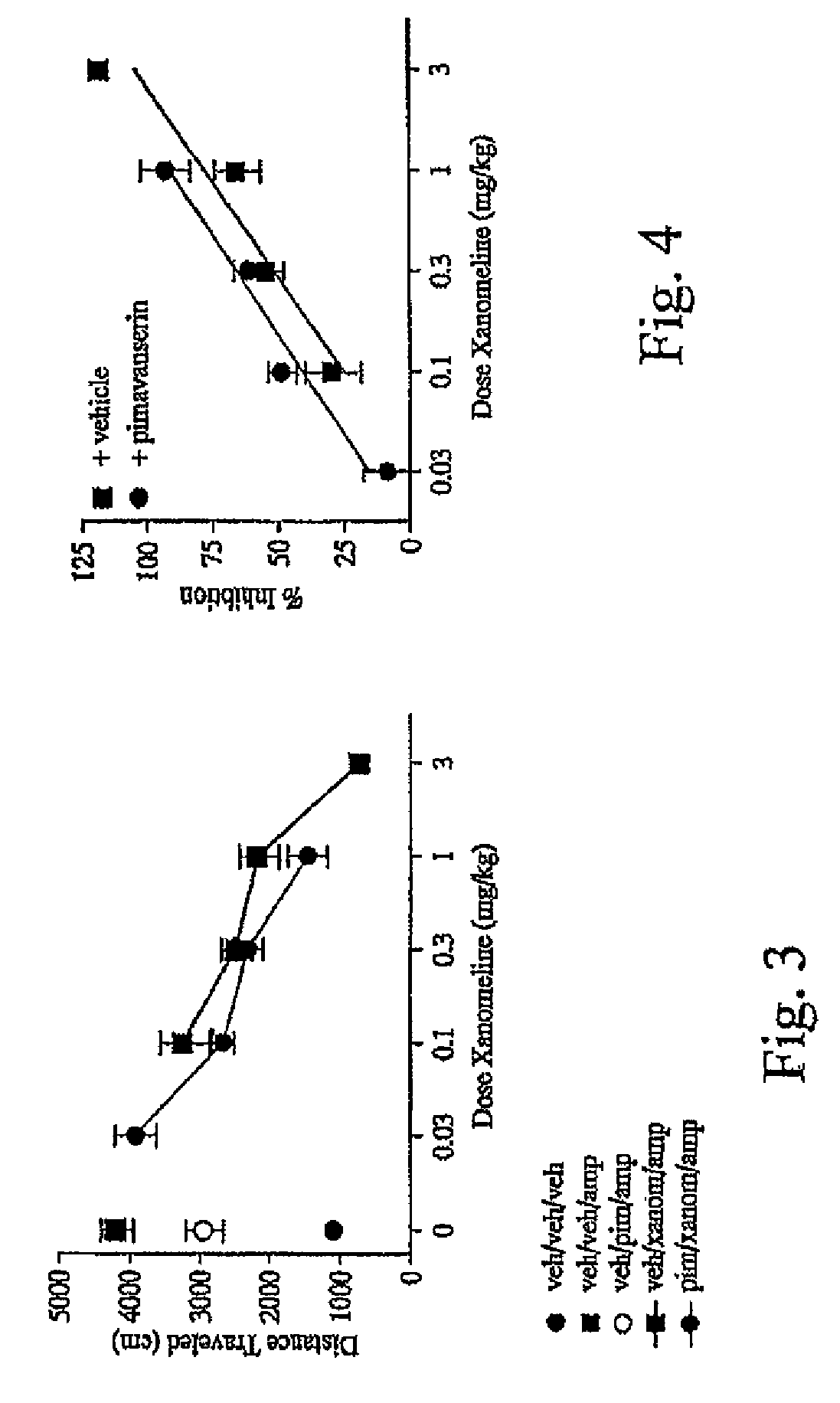
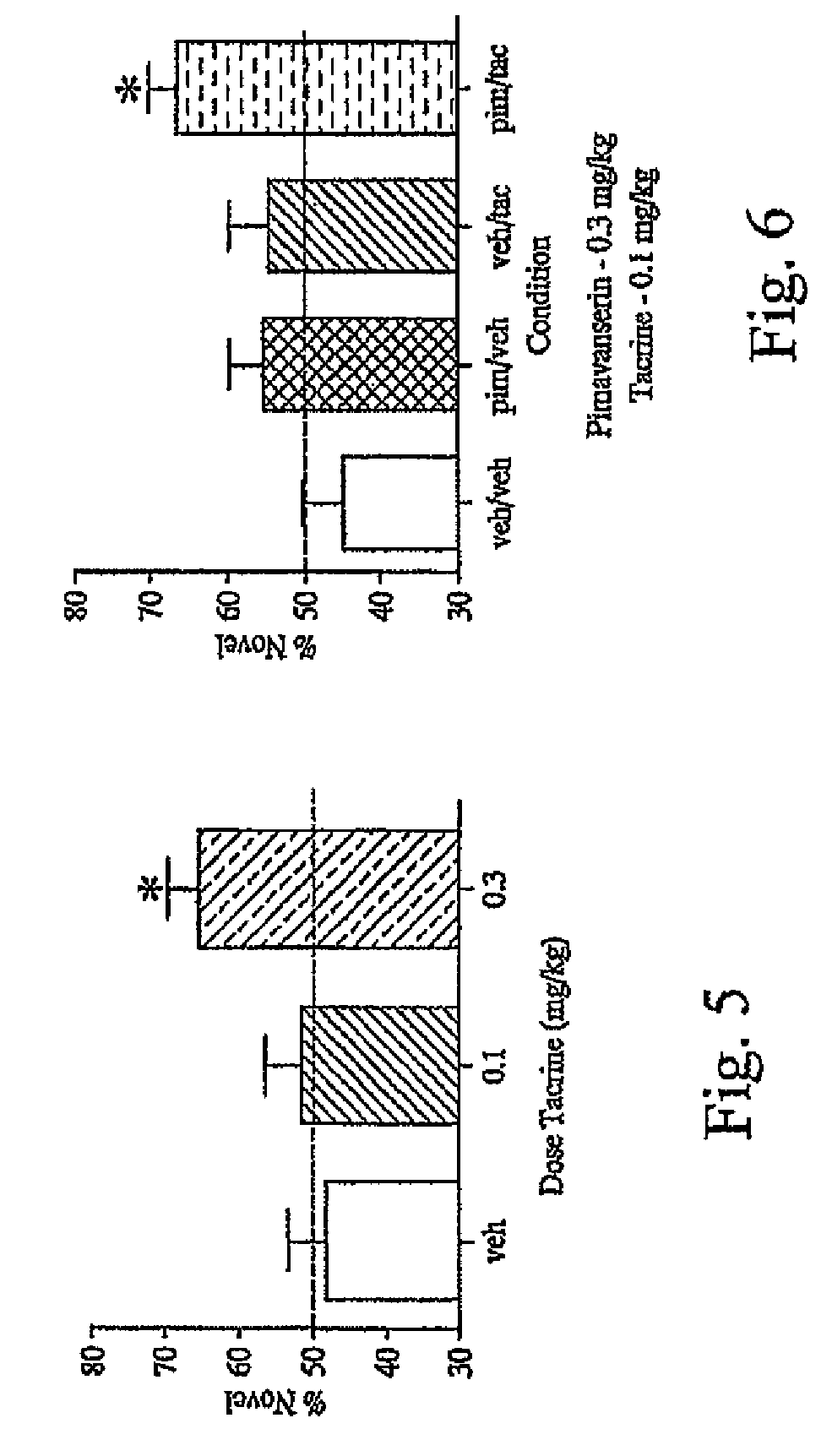
As disclosed herein, co-administration of pimavanserin with an agent that ameliorates one or more cholinergic abnormalities can have a synergistic effect on the efficacy of the agent. Disclosed herein are compositions which include pimavanserin in combination with an agent that ameliorates one or more cholinergic abnormalities. Also disclosed herein are methods for ameliorating or treating a disease condition characterized by one or more cholinergic abnormalities that can include administering pimavanserin in combination with an agent that ameliorates one or more cholinergic abnormalities.
Owner:ACADIA PHARMA INC
Co-administration of pimavanserin with other agents
As disclosed herein, co-administration of pimavanserin with an agent that ameliorates one or more cholinergic abnormalities can have a synergistic effect on the efficacy of the agent. Disclosed herein are compositions which include pimavanserin in combination with an agent that ameliorates one or more cholinergic abnormalities. Also disclosed herein are methods for ameliorating or treating a disease condition characterized by one or more cholinergic abnormalities that can include administering pimavanserin in combination with an agent that ameliorates one or more cholinergic abnormalities.
Owner:ACADIA PHARMA INC
Novel synthesis method for pimavanserin
ActiveCN105820110AEasy to crystallize and purifyHigh yieldOrganic chemistrySynthesis methodsPhosphoric acid
The invention provides a novel synthesis method for pimavanserin. The method comprises the steps that a compound formula (1a) is adopted as a raw material for preparing an active urea compound formula (1) and then subjected to a reaction with a compound formula (2) under an alkaline system to obtain a pimavanserin free alkali formula (3), and then pimavanserin free alkali forms a salt with tartaric acid to obtain a pimavanserin half tartrate formula (4). The method is simple in technological path, low in cost and suitable for industrial production. The formula is shown in the description, wherein HmA is the general formula of m-basic acid, HnA is the general formula of n-basic acid, m and n are 1 or 2 or 3, and the m-basic acid and the n-basic acid are selected from sulfuric acid, hydrochloric acid, phosphoric acid, p-toluenesulfonic acid, benzenesulfonic acid, methanesulfonic acid, methanoic acid, acetic acid, trifluoroacetic acid, oxalic acid, citric acid or tartaric acid.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
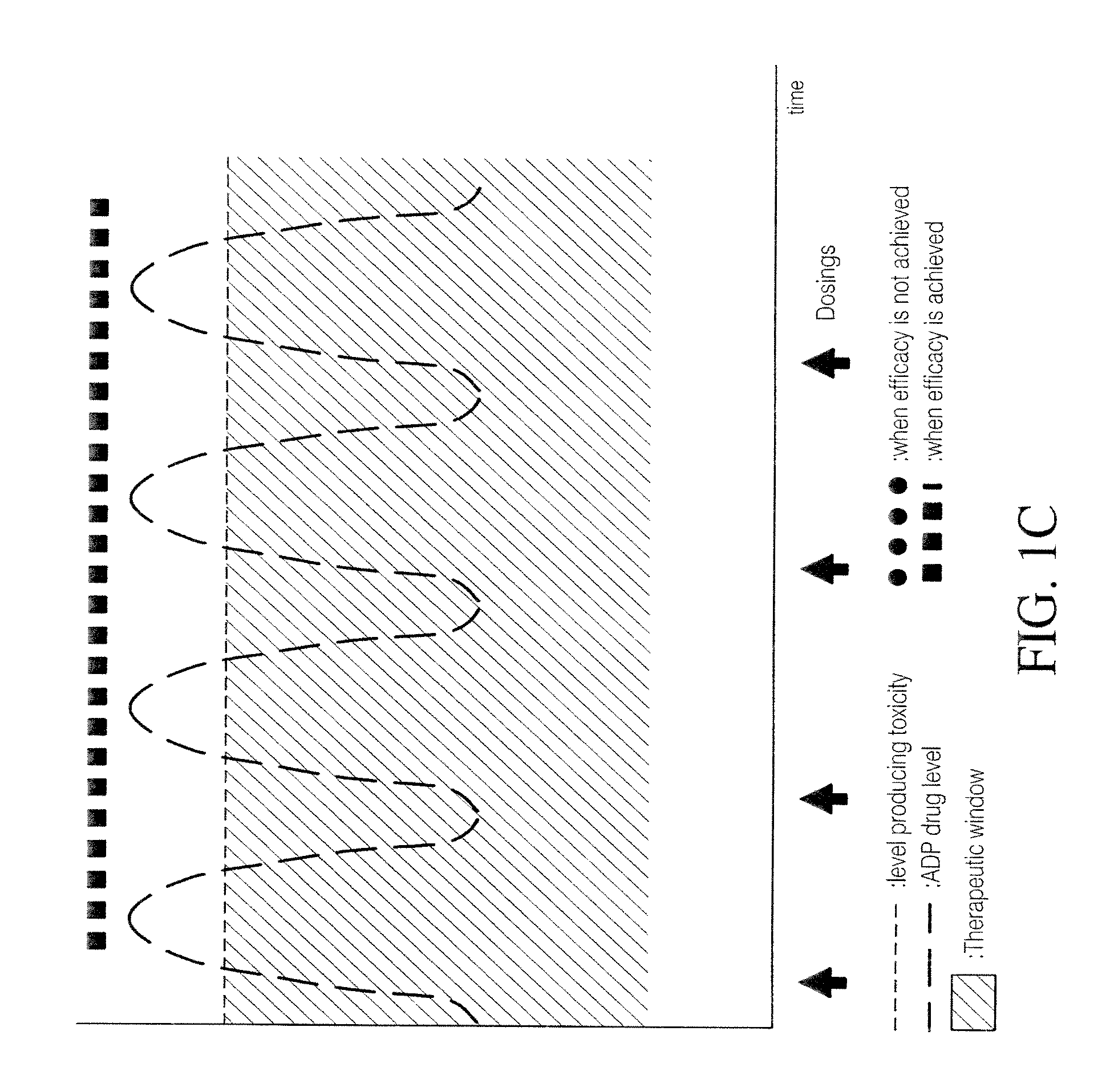
Processes and intermediates for the preparation of pimavanserin
ActiveUS20180037549A1Improve efficiencyQuality improvementNervous disorderCarbamic acid derivatives preparationPhotochemistryPimavanserin
Owner:PLIVA HRVATSKA D O O
Method for safely preparing pimavanserin and tartrate thereof by utilizing triphosgene
ActiveCN108947891AReduce the risk of safety productionReduce usageOrganic compound preparationOrganic chemistry methodsTartratePhosgene
The invention discloses a method for safely preparing pimavanserin and tartrate thereof by utilizing triphosgene. The synthetic route is as shown in the specification. The raw materials used by the method disclosed by the invention are safe, the cost is low, and the production cost is effectively reduced. The method disclosed by the invention is mild in reaction conditions, usage of phosgene thatis high in toxicity and difficult to operate can be avoided, and industrial production is easily realized.
Owner:LIVZON NEW NORTH RIVER PHARMA
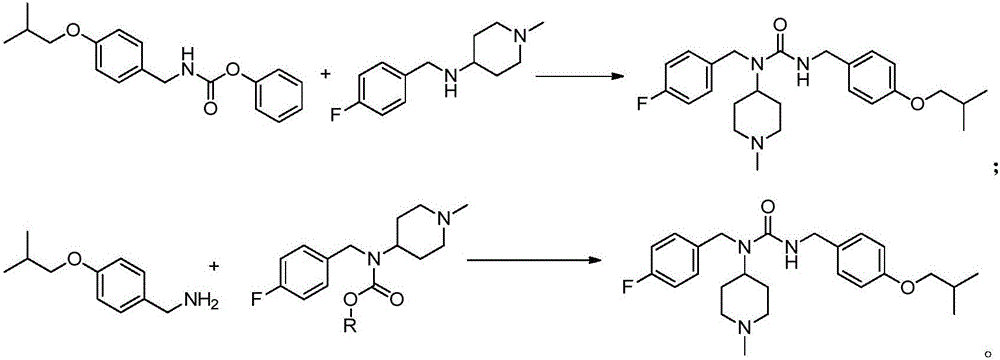
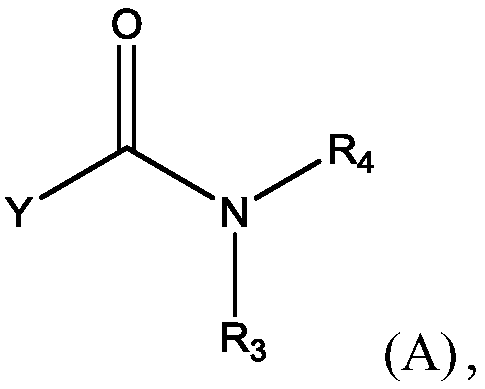
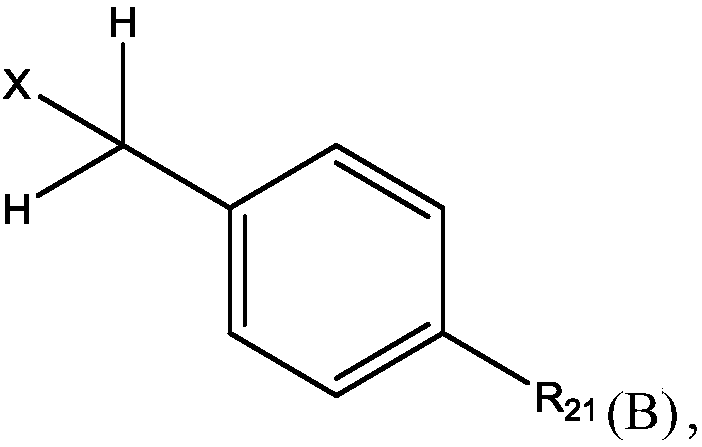
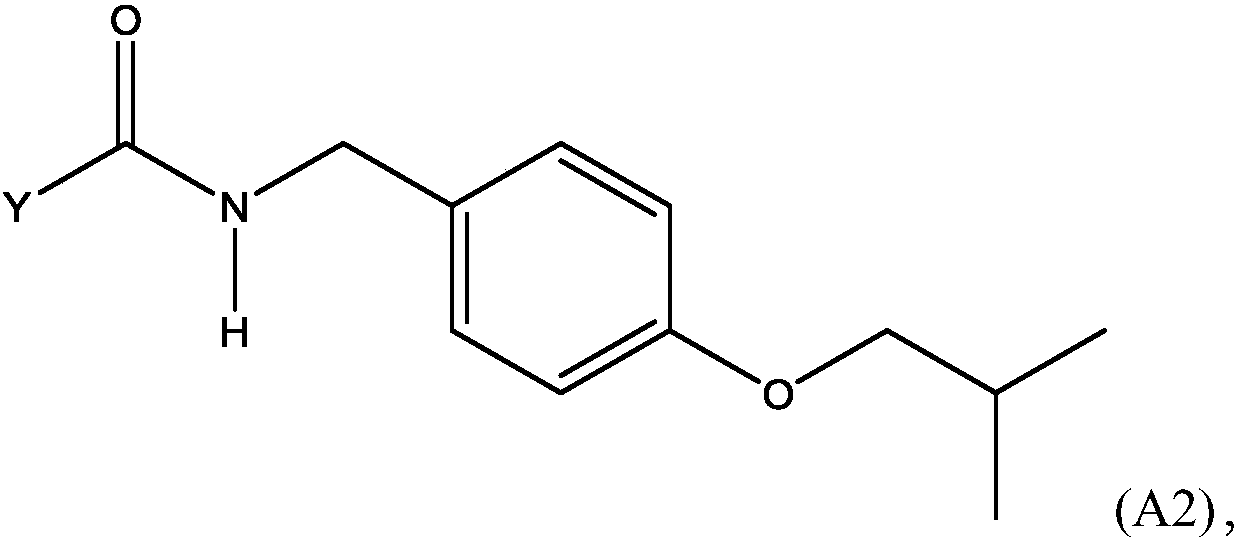
Methods for preparing n-(4-fluorobenzyl)-n-(1-methylpiperidin-4-yl)-n'-(4-(2-methylpropyloxy)phenylmethyl)carbamide and its tartrate salt and polymorphic form c
ActiveCN107848972AOrganic active ingredientsOrganic chemistry methodsCombinatorial chemistryTartrate
Disclosed herein are methods for obtaining N-(4-fluorobenzyl)-N-(l-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (pimavanserin) comprising the step of contacting an intermediate according to Formula (A) or a salt thereof, with an intermediate Formula B, or a salt thereof, to produce pimavanserin or a salt thereof wherein Y is -ORi or -NR2aR2b; R3 is hydrogen or substitutedor unsubstituted heteroalicyclyl, R4 is substituted or unsubstituted aralkyl; X is -OR22 or -NR23R24; (wherein R22 is hydrogen or substituted or unsubstituted C1-6alkyl and one of R23 and R24 is hydrogen and the other is hydrogen or N- methylpiperidin-4-yl); and R21 is -OCH2CH(CH3)2 or F; Also disclosed herein is the tartrate salt of N-(4-fluorobenzyl)-N-(l-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide and methods for obtaining the salt.
Owner:ACADIA PHARMA INC
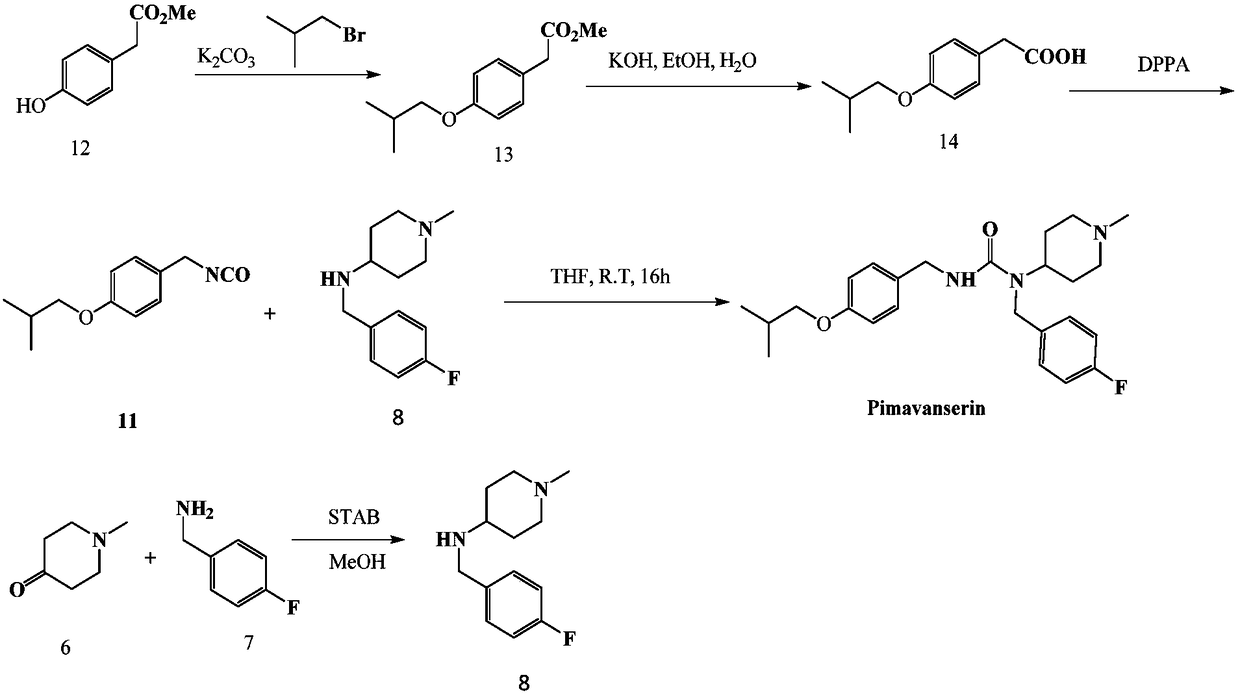
Preparation method of pimavanserin intermediate
InactiveCN105906531AThe reaction process is mild and controllableEasy post-processingIsocyanic acid derivatives preparationOrganic compound preparationToxic chemicalCarbonate
The invention relates to a preparation method of a pimavanserin intermediate which is 1-isobutoxy-4-isocyanatomethylbenzene. The preparation method comprises that 4-isobutoxyphenylmethanamine as an initial raw material and a bis(trichloromethyl)carbonate solution undergo a reaction under appropriate conditions to produce a desired product. The preparation method is free of highly toxic chemicals, has stable processes and a high yield and is suitable for industrial production.
Owner:HARVEST PHARMA HUNAN CO LTD
Pimavanserin preparation method
ActiveCN106588753AHigh yieldSuitable for industrial productionOrganic compound preparationAmino-hyroxy compound preparationRaw materialChemistry
The present invention relates to a pimavanserin preparation method, wherein 4-isobutoxybenzaldehyde is adopted as a starting raw material, the reaction condition is mild, the gas reaction material is avoided, the product yield is high, the process operation is simple, the special equipment request does not exist, and the method is especially suitable for industrial production.
Owner:CHONGQING PHARMA RES INST
Pimavanserin intermediate and preparation method of pimavanserin
InactiveCN108358817AThe reaction steps are simpleLower synthesis costCarbamic acid derivatives preparationOrganic compound preparationState of artPhosgene
The invention discloses a pimavanserin intermediate and a preparation method of pimavanserin. A structural general formula of the pimavanserin intermediate is shown in the description; the pimavanserin intermediate is prepared by carrying out reductive amination on 4-isobutoxybenzaldehyde and carbamate; a synthesis route is shown in the description; the pimavanserin is obtained through ammonolysisreaction. According to the preparation method of the pimavanserin intermediate, the target intermediate is obtained in one step through reductive amination reaction and reaction steps of the pimavanserin are extremely simplified; used raw materials are safe and the cost is low; reaction conditions are moderate and phosgene which has great toxicity and is uneasy to operate is not used, so that thepreparation method is easy to realize in industry; the intermediate and a product are easy to separate and purify, and the next-step reaction can be directly carried out to prepare the pimavanserin,without the need of separating the pimavanserin; the preparation method is simple to operate and the yield is higher than that of the prior art; the synthesis cost of the pimavanserin is reduced.
Owner:LIVZON NEW NORTH RIVER PHARMA
Preparation method of pimavanserin
The invention discloses a preparation method of pimavanserin. The preparation method comprises the following steps of mixing 1-(4-fluorobenzyl)-1-(4-methylpiperidyl)urea, 4-isobutoxybenzaldehyde and tetraisopropyl titanate in a solvent, reducing through hydroborate, and then obtaining the pimavanserin through purification. A synthesis method provided by the invention is succinct and high-efficiency, mild in reaction condition, easy to control and high in yield.
Owner:FUJIAN INST OF MICROBIOLOGY
Preparation method of pimavanserin key intermediate
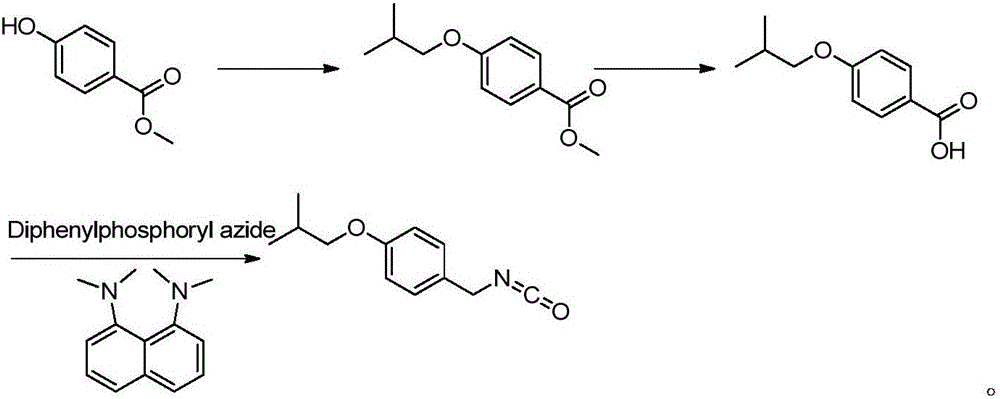
ActiveCN108794351AReduce the risk of safety productionReduce usageIsocyanic acid derivatives preparationCarboxylic acid nitrile preparationToxicityChemistry
The invention discloses a preparation method of a pimavanserin key intermediate. A synthesis route comprises a process shown in the description. According to the method disclosed by the invention, used raw materials are safe and the cost is low, so that the production cost is effectively reduced. The method disclosed by the invention has moderate reaction conditions and the utilization of phosgenewhich has great toxicity and is not easy to operate can be avoided, so that the method is easy to realize in industry.
Owner:LIVZON NEW NORTH RIVER PHARMA
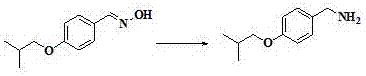
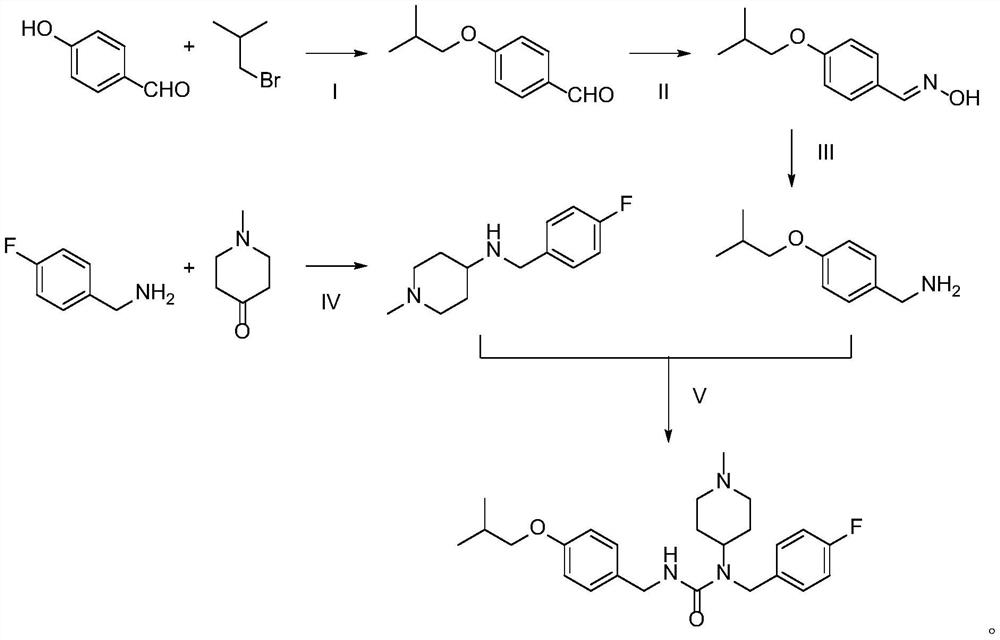
Preparation method of Pimavanserin
The invention discloses a preparation method of Pimavanserin. The preparation method comprises the following steps: (1) adopting 4-isobutoxy benzaldehyde to react with hydroxylamine hydrochloride in an alkaline liquor to prepare 4-isobutoxy benzaldoxime; (2) adopting a reducing agent to reduce the 4-isobutoxy benzaldoxime so as to generate 4-isobutoxy benzylamine; (3) adopting a carbonyl compound to react with the 4-isobutoxy benzylamine to prepare N-(4-isobutoxy benzyl) phenyl carbamate; and (4) adopting the N-(4-isobutoxy benzyl) phenyl carbamate to react with N-(4-fluorobenzyl)-1-methyl piperidine-4-amine, and thus obtaining the Pimavanserin. Heating is not needed when the 4-isobutoxy benzaldoxime is prepared in the method, so that the energy consumption is reduced; zinc powder is used for replacing hydrogen and palladium carbon so as to generate the 4-isobutoxy benzylamine, and low-cost easily-obtained diphenyl carbonate is used for replacing hypertoxic carbonyl chloride and expensive DPPA, so that the safety is improved, and the environmental protection is facilitated.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
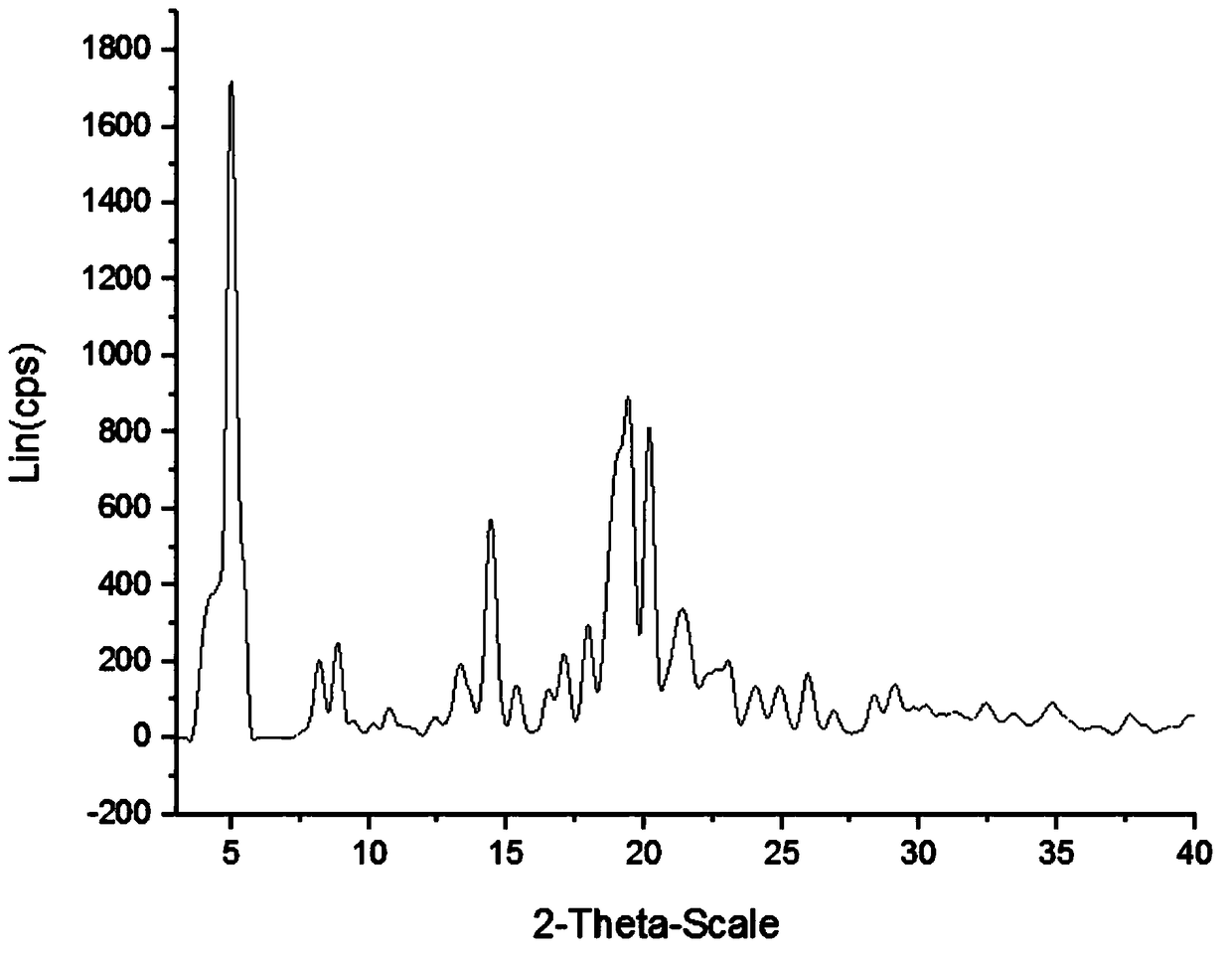
Method for preparing crystal form B of pimavanserin semi-tartrate
InactiveCN106946764AHigh purityIncrease productivityOrganic chemistry methodsEconomic benefitsSolvent
The invention discloses a method for preparing a crystal form B of pimavanserin semi-tartrate. The method comprises the following steps of adding the pimavanserin semi-tartrate into absolute methanol, stirring, heating, and dissolving; cooling, crystallizing, filtering, and drying, so as to obtain the crystal form B of the pimavanserin semi-tartrate, wherein the weight and volume ratio of the pimavanserin semi-tartrate to the absolute methanol is 1:(4 to 5)g / ml. The method has the advantages that by using the absolute methanol as the solvent, the crystal form B of the pimavanserin semi-tartrate is successfully prepared; the method is simple and convenient, the production efficiency is high, the economic benefit is good, and the method is suitable for industrialized production.
Owner:CHENGDU BAIYU PHARMA CO LTD
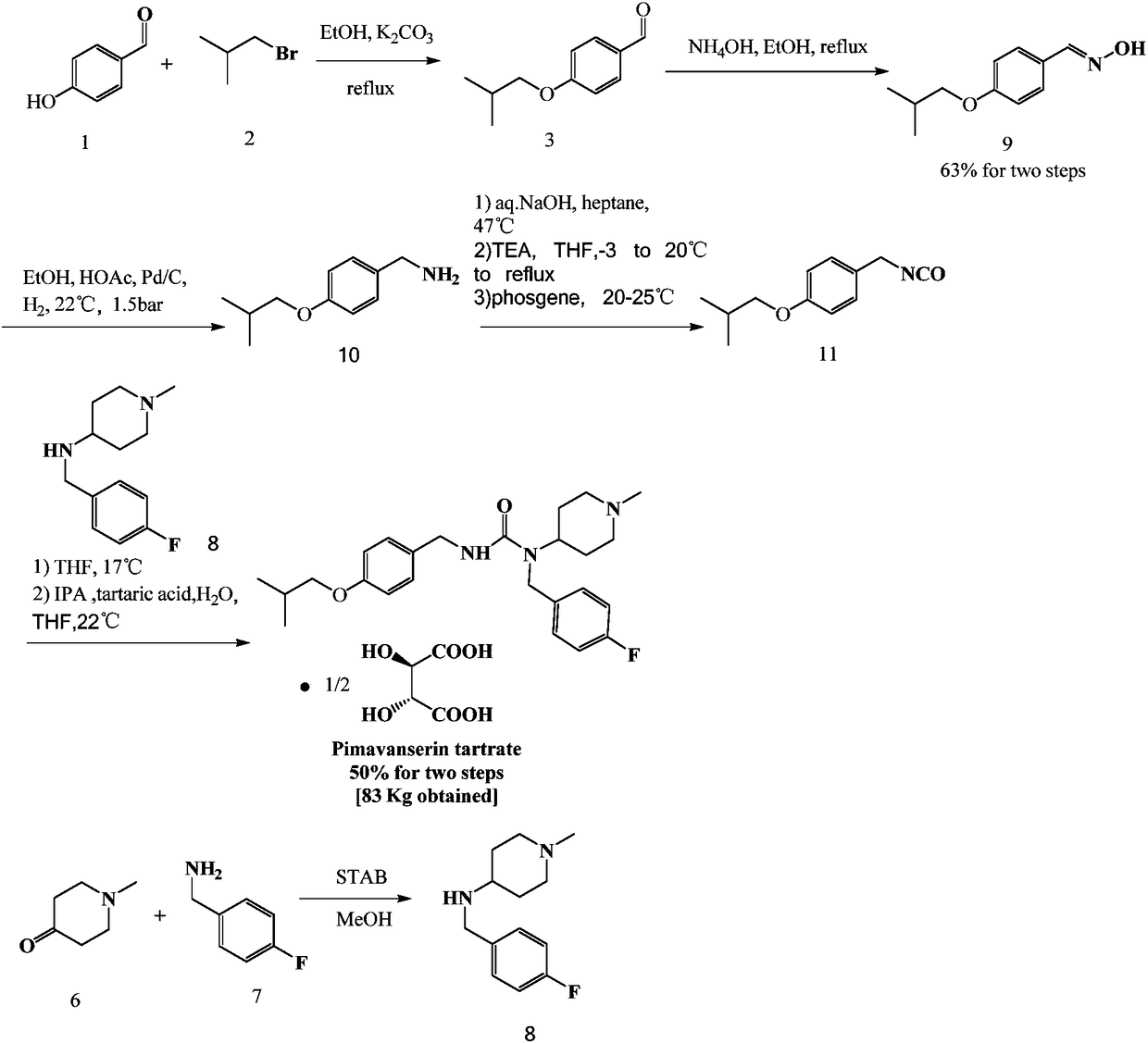
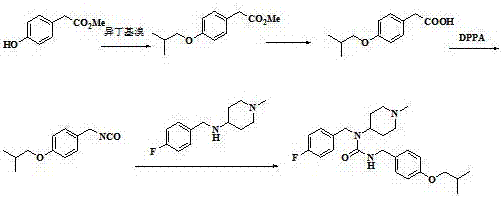
Method for preparing pimavanserin and tartrate thereof
The invention provides an improved method for preparing pimavanserin and tartrate thereof. The method comprises the following steps: taking a compound 4-(4-fluorobenzylamino)-1-methylpiperidine as a starting material and carrying out acylation reaction on the starting material and triphosgene in the presence of triethylamine to prepare a compound (4-fluorobenzyl)-(1-methylpiperidine-4-yl)carbamyl chloride; then taking the (4-fluorobenzyl)-(1-methylpiperidine-4-yl)carbamyl chloride and 4-isobutoxybenzylamine to react in the presence of triethylamine, so as to prepare the pimavanserin and the tartrate thereof. The method provided by the invention is simple, efficient and economical and the quality of an intermediate is controllable, so that the method is suitable for industrial preparation.
Owner:SUNSHINE LAKE PHARM CO LTD
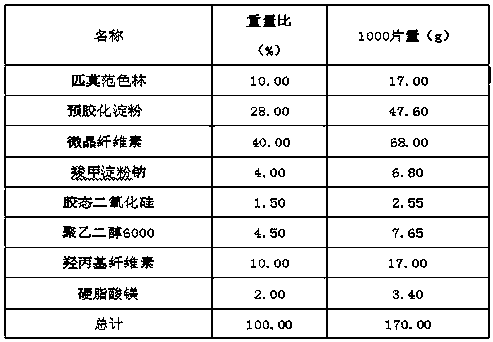
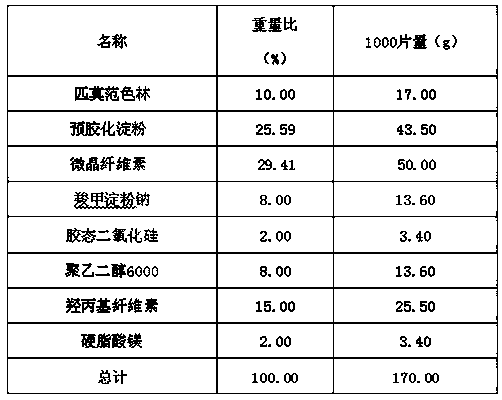
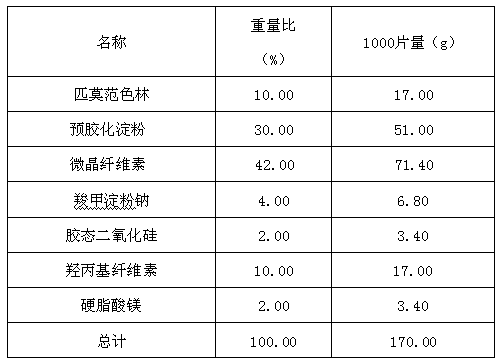
Pimavanserin tablet and preparation method thereof
InactiveCN109568278ADissolution stabilityReduce static electricityOrganic active ingredientsNervous disorderLow-substituted hydroxypropylcellulosePolyethylene glycol
The invention belongs to the field of pharmaceutic preparation, and particularly relates to a pimavanserin tablet and a preparation method thereof. The pimavanserin tablet is composed of active components of pimavanserin, colloidal silicon dioxide, polyethylene glycol, a filling agent selected from dextrin, corn starch, pregelatinized starch, cellulose microciystalline, mannitol and lactose, a disintegrating agent selected from carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropyl cellulose and crospovidone, and a lubricating agent selected from magnesium stearate,talcum powder, and sodium stearyl fumarate. The pimavanserin tablet has good stability, and can significantly improve drug dissolution and bioavailability.
Owner:BEIJING VENTUREPHARM BIOTECH
Tartaric acid pimavanserin oral instant film and preparation method thereof
InactiveCN106265605AGreat tasteEasy to prepareOrganic active ingredientsNervous disorderMedicineDysphagia
The invention belongs to the field of medicine preparations and relates to a tartaric acid pimavanserin oral instant film and a preparation method thereof. The tartaric acid pimavanserin oral instant film is good in taste, can be quickly dissolved in the oral cavity when taken without water, and is particularly suitable for the aged and patients suffering from dysphagia. A preparation process of the tartaric acid pimavanserin oral instant film is simple and easy to implement, auxiliary materials are common and moderate in price, and industrial prospects are good. Each tartaric acid pimavanserin oral instant film contains 20 mg of tartaric acid pimavanserin. The invention further provides the preparation process of the tartaric acid pimavanserin oral instant film.
Owner:天津市聚星康华医药科技有限公司
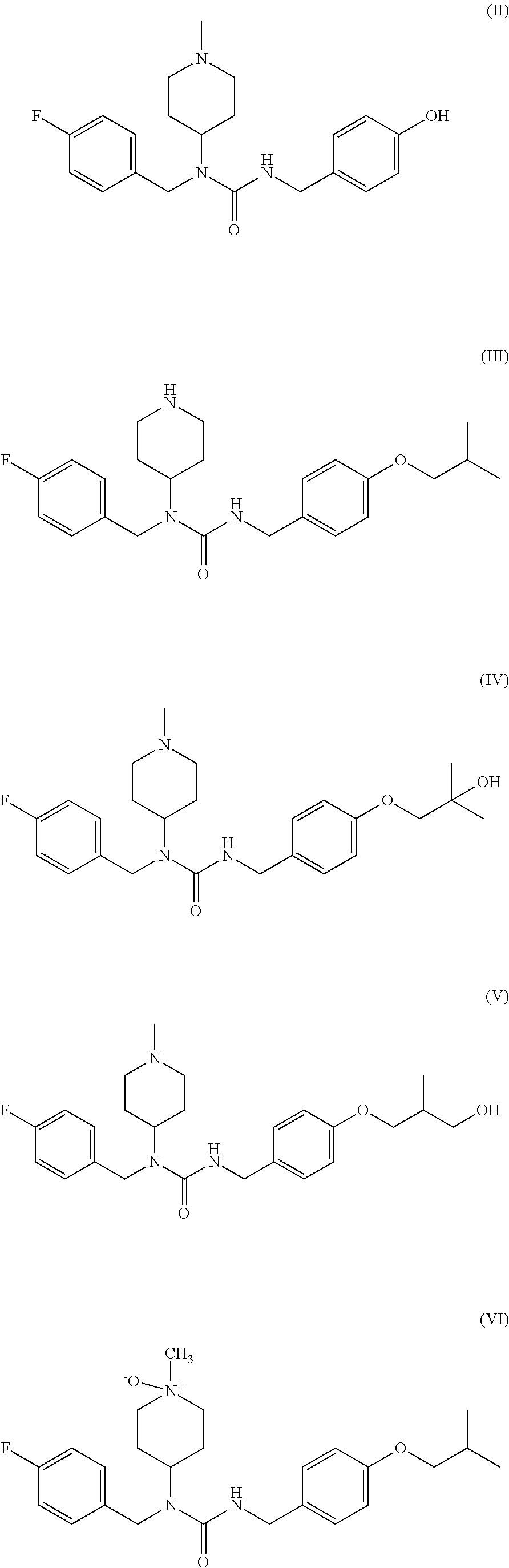
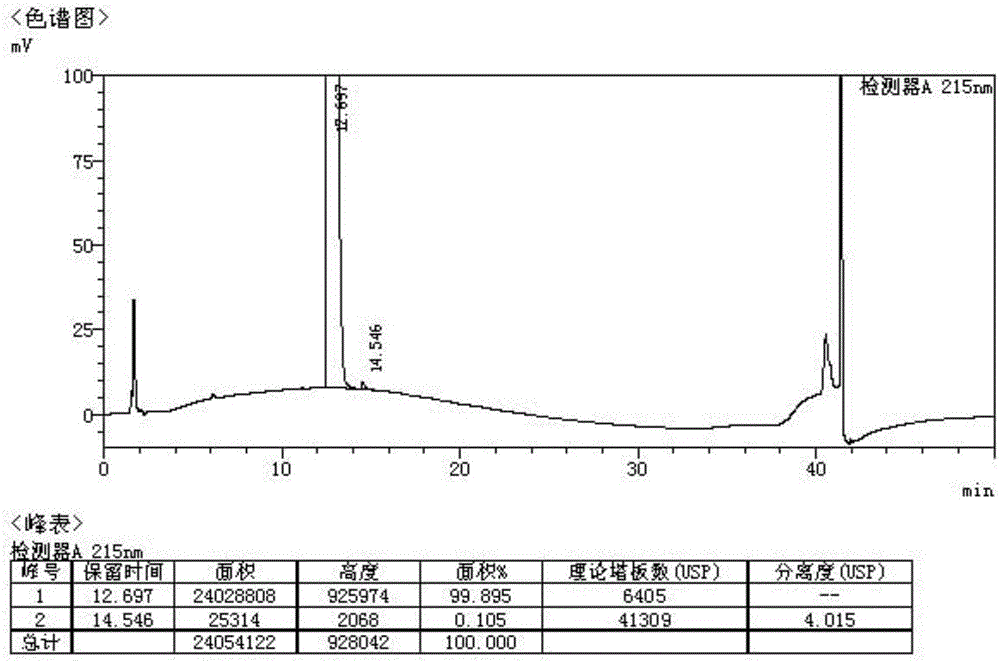
Tartaric acid pimavanserin impurities and preparation method thereof
ActiveCN107216271AUrea derivatives preparationCarbamic acid derivatives preparationMedicinal chemistryImpurity
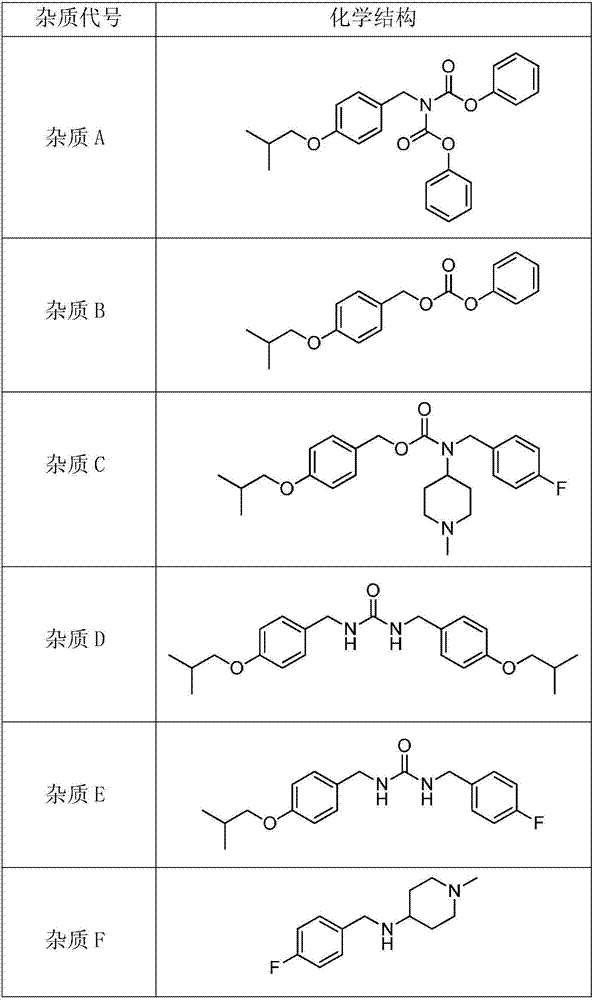
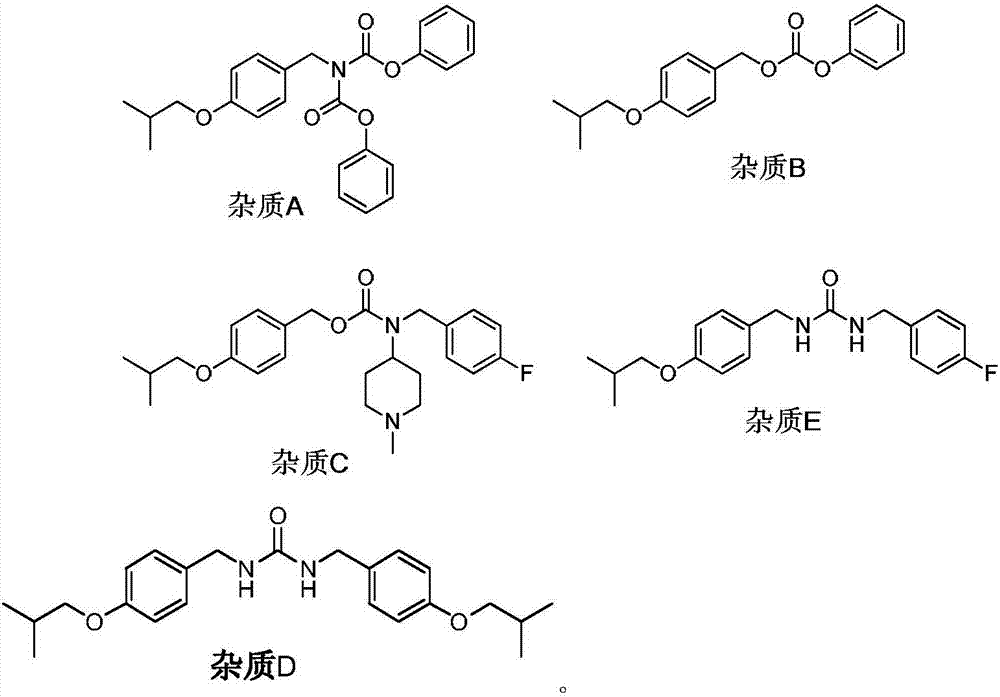
The invention discloses tartaric acid pimavanserin impurities, namely N-(4-isobutoxy benzyl) diphenyl aminodiphthalate (impurity A), 4-isobutoxy benzyl carbonic acid phenyl ester (impurity B), 4-fluorobenzyl (1-methylpiperidine-4-yl) carbonic acid-4-isobutoxy benzyl ester (impurity C) and N-( isobutoxy benzyl)-N'-(4-fluorobenzyl) urea (impurity E). In addition, the invention further discloses a preparation method of tartaric acid pimavanserin impurities A, B, C and E and an N,N'-bis(4-isobutoxy benzyl) urea (impurity D). Through application of the tartaric acid pimavanserin impurities as reference substances in research on the quality of the tartaric acid pimavanserin intermediate, a crude drug and a compound preparation thereof, a solid foundation is laid for research on the quality of tartaric acid pimavanserin.
Owner:SHENYANG PHARMA UNIVERSITY
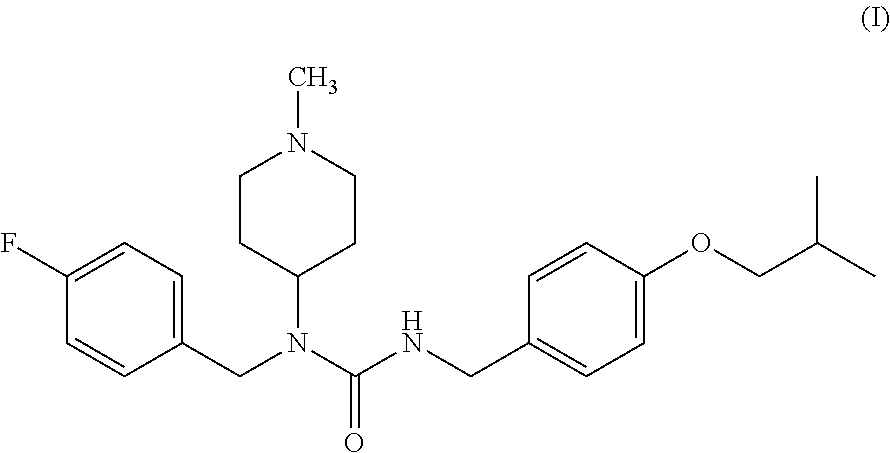
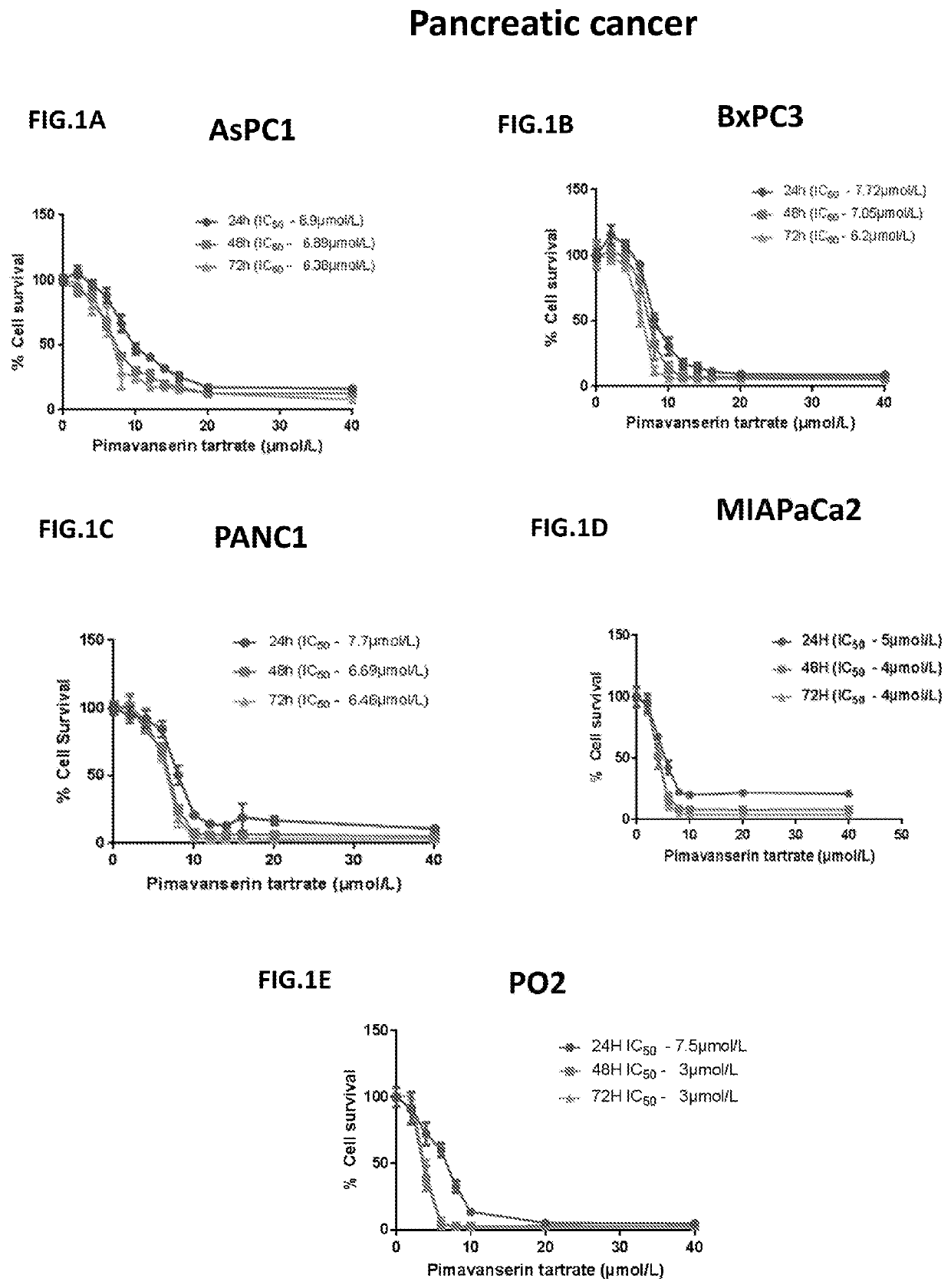
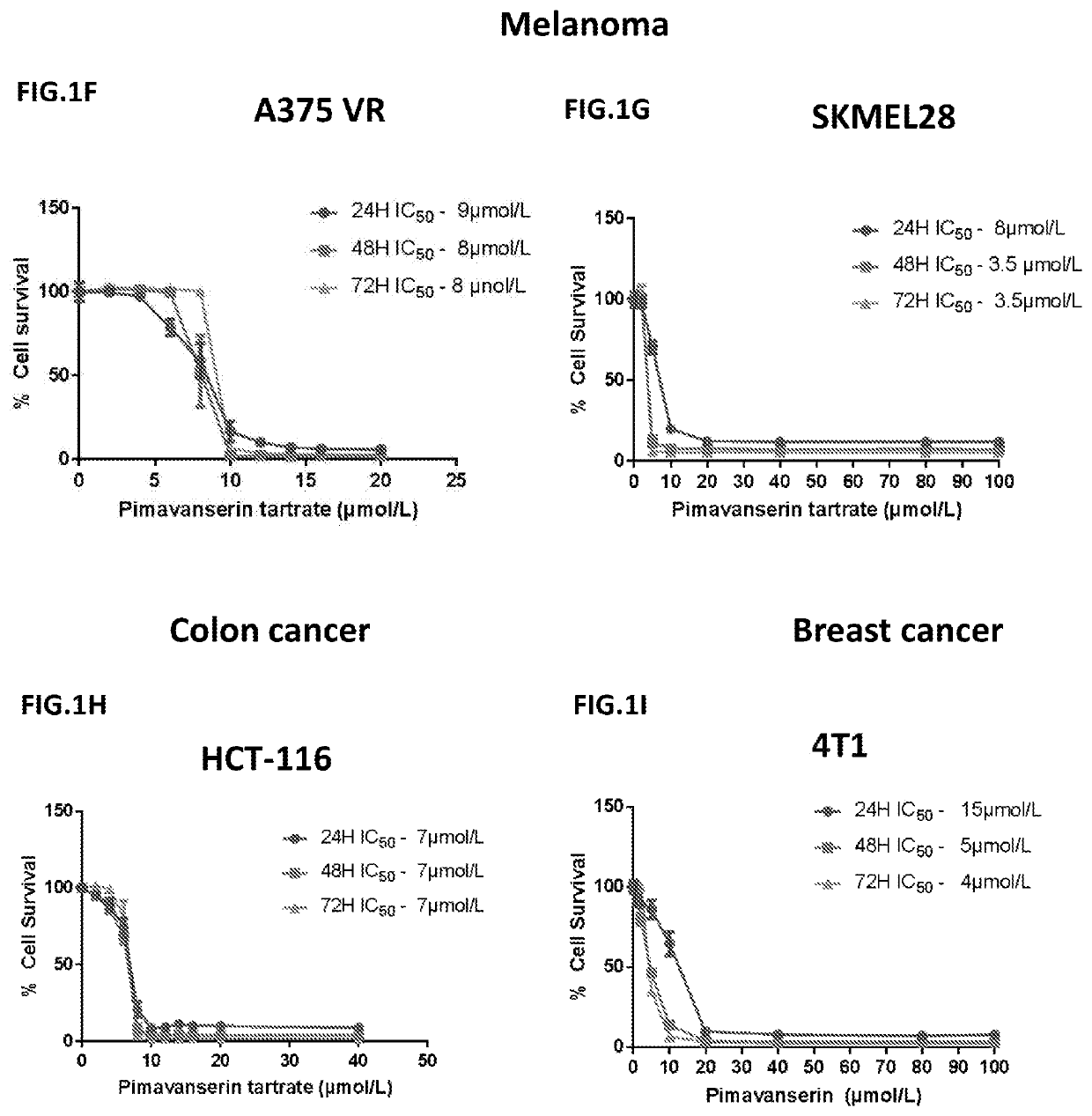
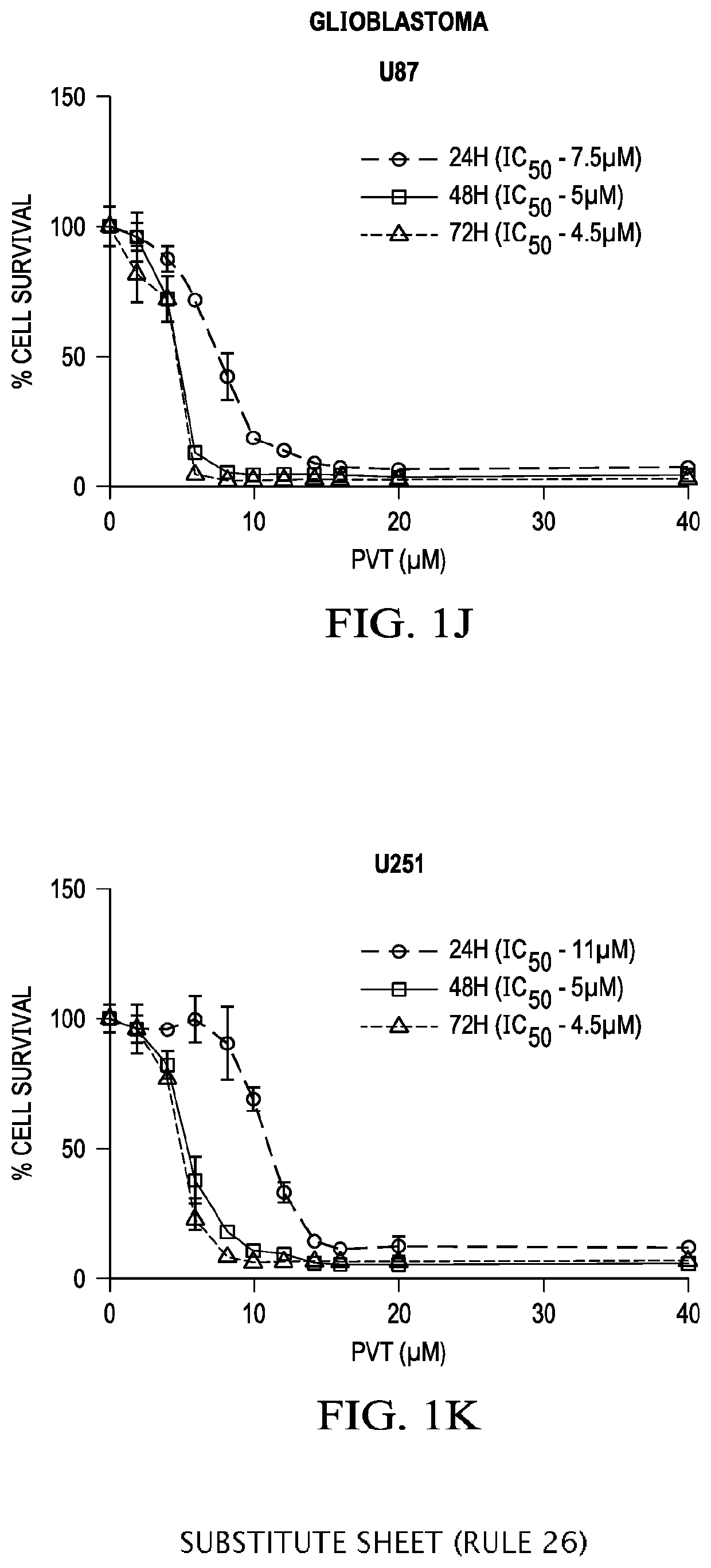
Application of pimavanserin in preparation of antitumor drugs
ActiveCN113274390AImprove securityLow toxicityOrganic active ingredientsOrganic chemistryPharmaceutical drugCombinatorial chemistry
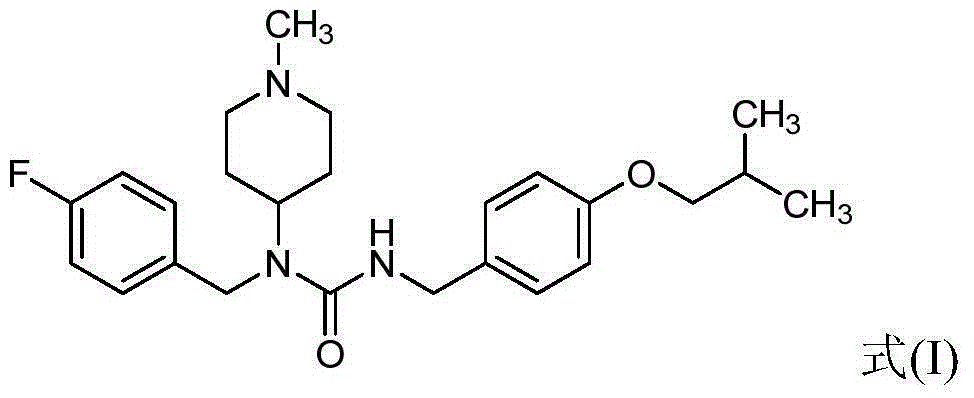
The invention relates to application of pimavanserin in preparation of antitumor drugs, belongs to the technical field of medicines, and particularly relates to novel application of pimavanserin in preparation of antitumor drugs. The structural formula of the pimavanserin is shown in the formula I. The biological activity test of the compound shows that the compound has antitumor activity and is a novel antitumor drug.
Owner:SHENYANG PHARMA UNIVERSITY
Compositions and Methods for Treating Cancer
ActiveUS20200268732A1Reduce in quantityOrganic active ingredientsAntineoplastic agentsPharmaceutical drugPharmaceutical medicine
The present invention includes composition and methods for treating cancers comprising administering to a subject a pharmaceutical composition comprising Pimavanserin or derivatives thereof in an amount sufficient to treat the reproductive cancer in the subject and a pharmaceutically acceptable carrier.
Owner:TEXAS TECH UNIV SYST
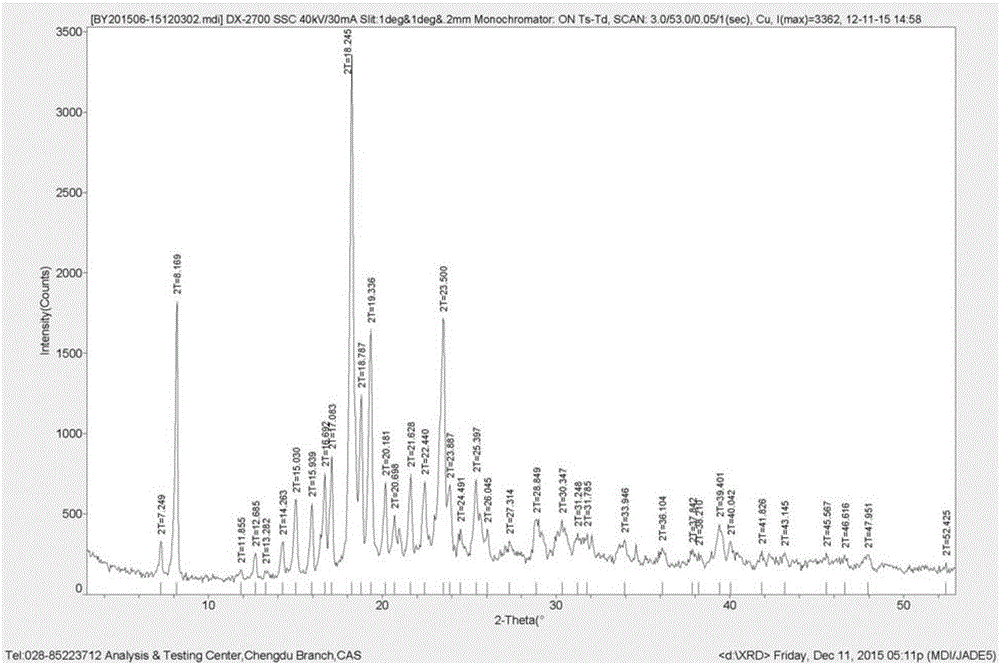
Preparation method of pimavanserin hemitartrate crystal form C
The invention discloses a preparation method of a pimavanserin hemitartrate crystal form C. The method includes the steps of: (1) under the protection of an inert gas, adding pimavanserin free alkali into butanone, and conducting dissolving, then adding tartaric acid to carry out reaction to obtain a reaction solution; (2) carrying out crystallization, filtering and drying on the reaction solution obtained in step (1), thus obtaining the pimavanserin hemitartrate crystal form C. The method adopts pimavanserin free alkali as the raw material, and successfully prepares the pimavanserin hemitartrate crystal form C. Compared with the prior art, the method provided by the invention is simple, safe and environment-friendly, shortens the stirring crystallization time from 3 days to 2-3 hours, greatly improves the production efficiency, has good economic benefits, and is very suitable for industrial production.
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for preparing pimavanserin crystal form C
The invention provides a method for preparing a pimavanserin crystal form C. The method comprises the step of: conducting reflux on a tartrate crystal A of N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-(2-methyl propoxy)-phenyl methyl) urea in acetone for 3-6 h to obtain the pimavanserin crystal form C. The invention creatively realizes the crystal form transformation of pimavanserin by simple heating and refluxing, does not need deoxidation treatment of solvent, or the protection of inert gas, or addition of seeds; and the operation steps are simple without harsh conditions, and are more conducive to industrialization.
Owner:LIANGJIANG MEDICINE CO LTD
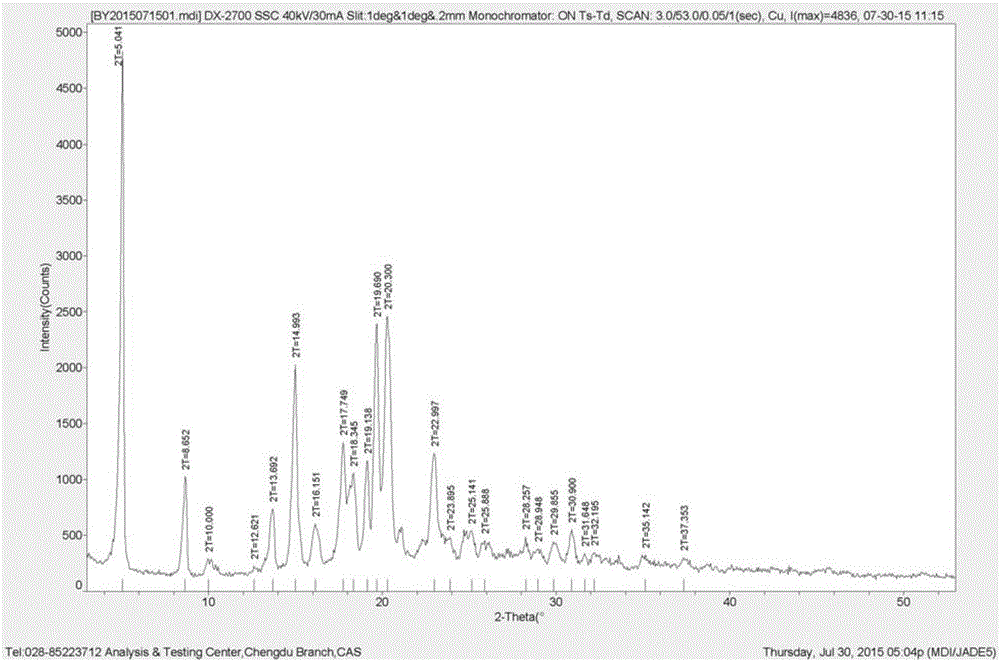
Method for preparing pimavanserin hemitartrate crystal form B
ActiveCN105859608ASimple methodIncrease productivityOrganic chemistry methodsEconomic benefitsSolvent
The invention discloses a method for preparing a pimavanserin hemitartrate crystal form B. The method comprises the following steps: adding pimavanserin hemitartrate into water; and after the pimavanserin hemitartrate is dissolved, concentrating and drying to obtain the pimavanserin hemitartrate crystal form B, wherein the weight-to-volume ratio of the pimavanserin hemitartrate to the water is 1:(4-6) g / ml. By using the nontoxic harmless water as the solvent, the pimavanserin hemitartrate crystal form B is successfully prepared. Besides, the method is simple and convenient, has the advantages of high safety, environment friendliness, high production efficiency and favorable economic benefits, and is very suitable for industrialized production.
Owner:CHENGDU BAIYU PHARMA CO LTD
Processes and intermediates for the preparation of Pimavanserin
ActiveUS10343993B2Good efficiency and qualityNervous disorderCarbamic acid derivatives preparationPhotochemistryPimavanserin
The present disclosure relates to novel, safe and efficient processes for the synthesis of Pimavanserin and salts thereof, as well as novel intermediates that can be used in these processes.
Owner:PLIVA HRVATSKA D O O
Preparation method for pimavanserin
The invention relates to a one-pot preparation method for pimavanserin. The method prepares pimavanserin (a compound IV) from 4-isobutoxybenzylamine as shown in a formula (II) or a salt thereof, a carbonylation reagent as shown in a formula (III) and N-(4-fluorobenzyl)-1-methylpiperidin-4-amine as shown in a formula (I) by using a one-pot process. The method is highly efficient, high in yield, low in cost, safe, environment friendly and suitable for industrial production, and has great application value.
Owner:SHANDONG SIMCERE BIO PHARMA CO LTD +2
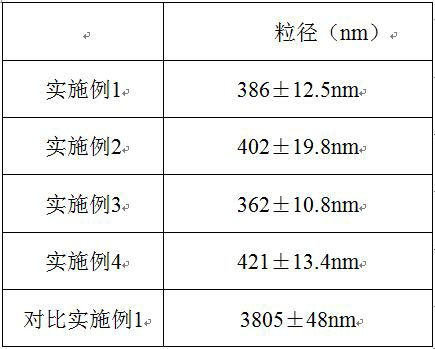
Pimavanserin nanocrystalline capsule and preparation method thereof
InactiveCN111821277AImprove external dissolutionImprove bioavailabilityOrganic active ingredientsNervous disorderDrugs preparationsPharmaceutical drug
The invention belongs to the field of pharmaceutical preparations and particularly relates to a pimavanserin nanocrystalline capsule and a preparation method thereof. The preparation method comprisesthe following steps: 1) adding pimavanserin and poloxamer 188 to water, and carrying out mixing to obtain a mixed solution; 2) homogenizing the mixed solution for 3 times to obtain a suspension; 3) performing spray drying on the suspension in a spray dryer; and 4) after mixing with other auxiliary materials, performing capsule filling. The prepared pimavanserin capsule can significantly increase the dissolution rate so as to improve the bioavailability of the medicine in vivo.
Owner:BEIJING VENTUREPHARM BIOTECH
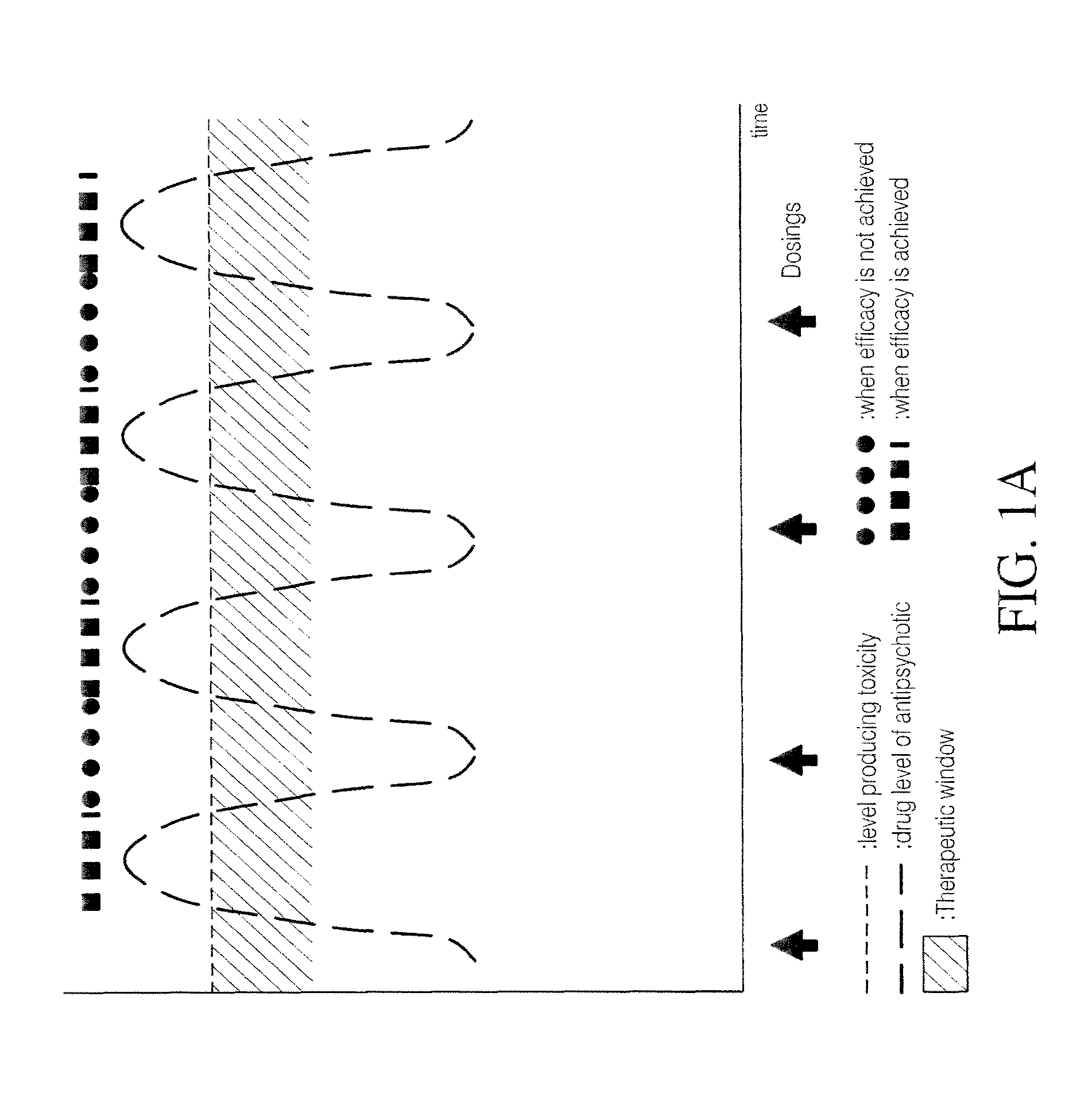
Combination of pimavanserin and risperidone for the treatment of psychosis
ActiveUS20130143901A1Reducing and preventing hyperprolactinemiaCompounds screening/testingBiocideSide effectAntipsychotic drug therapy
Combinations of 5-HT2A inverse agonists or antagonists such as pimavanserin with antipsychotics such as risperidone are shown to induce a rapid onset of antipsychotic action and increase the number of responders when compared to therapy with the antipsychotic alone. These effects can be achieved at a low dose of the antipsychotic, thereby reducing the incidence of side effects. The combinations are also effective at decreases the incidence of weight gain and increased glucose or prolactin levels caused by the antipsychotic.
Owner:ACADIA PHARMA INC
Solid oral pharmaceutical composition containing pimavanserin and preparation method for solid oral pharmaceutical composition
InactiveCN109498582ASimple prescriptionMature technologyOrganic active ingredientsNervous disorderOrally disintegrating tabletMedical prescription
The invention relates to a pharmaceutical composition containing pimavanserin and a preparation method for the pharmaceutical composition. According to the composition suitable for preparing the orally disintegrating tablet, active ingredients are contained, a disintegrating agent and a diluent are also contained, in addition, a proper amount of a flavoring agent, a lubricant and a flow aid can also be contained. The composition has the advantages of being simple in prescription and mature in process, and only common tablet production equipment is adopted. The pharmaceutical composition can berapidly disintegrated within 30 s in an oral cavity and is not required to be taken with water, the medication compliance of Parkinson's disease is improved, and the economic loss caused by drug discarding by a patient can be avoided.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com