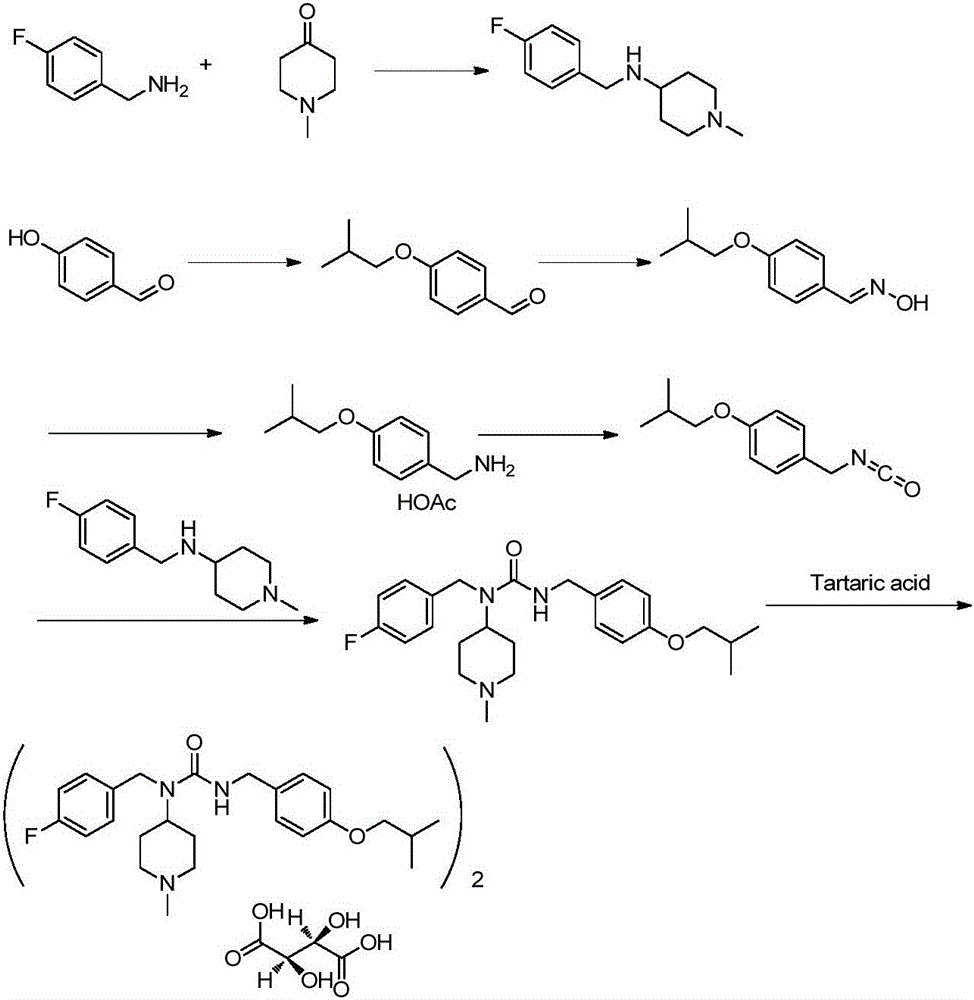
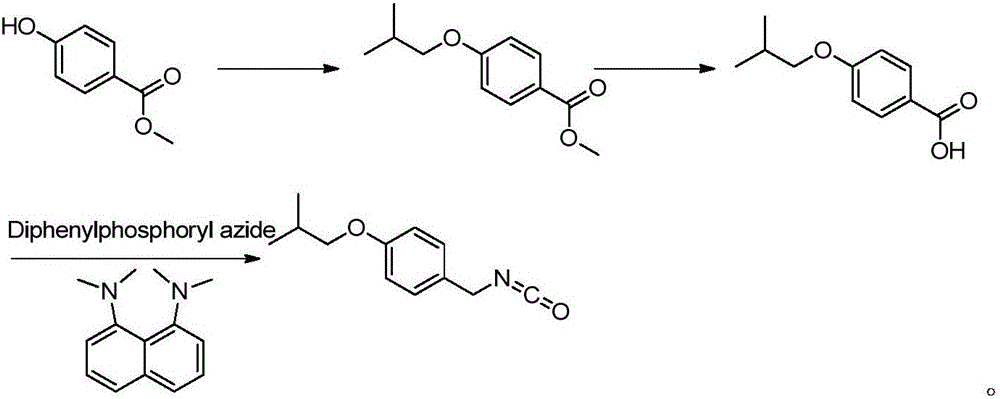
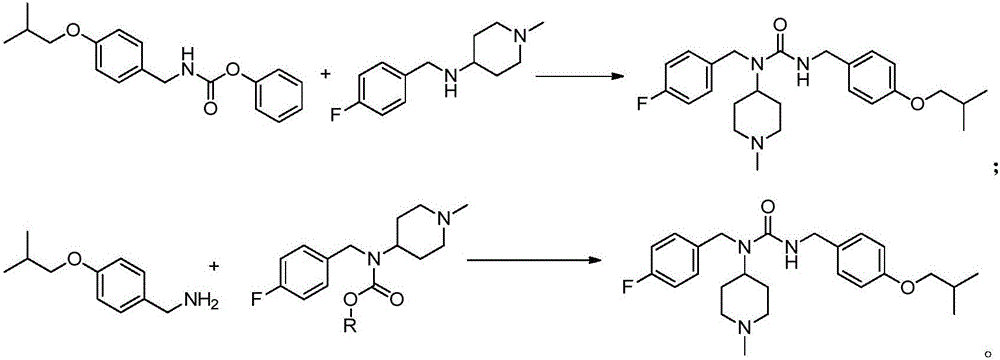
Novel synthesis method for pimavanserin
A new synthesis technology of pimavanserin, which is applied in the direction of organic chemistry, can solve the problems of optimization of starting material synthesis, difficulty in product purification, and low experimental efficiency, and achieve shortened reaction steps, high product purity, and simple experimental operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049]Add 4-fluorobenzaldehyde (12.41g, 100mmol), 4-amino-1-methylpiperidine (11.42g, 100mmol), and dichloromethane (124mL) into a three-necked flask, stir for 1-2 hours, then add acetic acid 25 mL, add sodium acetate borohydride (42.39 g, 200 mmol) in batches, react at room temperature for 6-8 hours, add 100 mL of water to quench the reaction after the reaction, and add 10% sodium hydroxide solution to adjust the pH value to 9-10, and separate , the aqueous phase was extracted twice with dichloromethane (143mL), the combined organic phase was washed twice with saturated brine (143mL), dried over sodium sulfate, and concentrated to obtain an oily product, which was dissolved with isopropanol (200mL) and stirred, heated to 55-60 ℃, 85% phosphoric acid (8.07g, 70mmol) was added dropwise, a small amount of seed crystals were added, the crystallization was carried out at room temperature, filtered, and dried to obtain compound 1a-1 (23.87g, 83%). ESIm / z=223.2(M+1), 1 ...
Embodiment 2
[0051]
[0052] Add 4-fluorobenzaldehyde (12.41g, 100mmol), 4-amino-1-methylpiperidine (11.42g, 100mmol), and dichloromethane (124mL) into a three-necked flask, stir for 1-2 hours, then add acetic acid 25mL, add sodium borohydride (7.57g, 200mmol) in batches, react at room temperature for 6-8 hours, add 100mL of water to quench the reaction after the reaction, and add 10% sodium hydroxide solution to adjust the pH value to 9-10, separate the liquid, The aqueous phase was extracted twice with dichloromethane (143mL), the combined organic phase was washed twice with saturated brine (143mL), dried over sodium sulfate, and concentrated to obtain an oily product which was dissolved by adding tetrahydrofuran (200mL) and stirred, heated to 45-55°C, and dripped Add methanesulfonic acid (19.22g, 200mmol), add a small amount of seed crystals, drop to room temperature for crystallization, filter and dry to obtain compound 1a-2 (29.84g, 72%).
[0053] ESIm / z=223.2(M+1), 1 HNMR(DMSO-d6...
Embodiment 3
[0055]
[0056] Add 4-fluorobenzaldehyde (12.41g, 100mmol), 4-amino-1-methylpiperidine (11.42g, 100mmol), and dichloromethane (124mL) into a three-necked flask, stir for 1-2 hours, then add acetic acid 25mL, add sodium borohydride (7.57g, 200mmol) in batches, react at room temperature for 6-8 hours, add 100mL of water to quench the reaction after the reaction, and add 10% sodium hydroxide solution to adjust the pH value to 9-10, separate the liquid, The aqueous phase was extracted twice with dichloromethane (143mL), and the combined organic phase was washed twice with saturated brine (143mL), dried over sodium sulfate, concentrated to obtain an oily product, added ethyl acetate (200mL), stirred and dissolved, and heated to 45-55°C , dropwise added acetic acid (12.01g, 200mmol), added a small amount of seed crystals, cooled down to room temperature for crystallization, filtered, and dried to obtain compound 1a-3 (27.05g, 79%).
[0057] ESIm / z=223.2(M+1), 1 HNMR(DMSO-d6,400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com