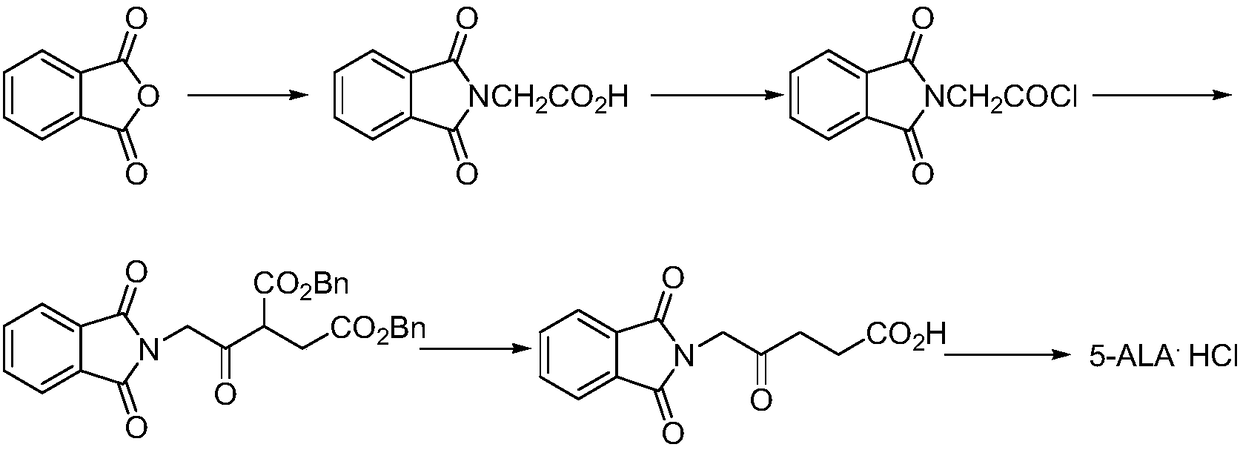
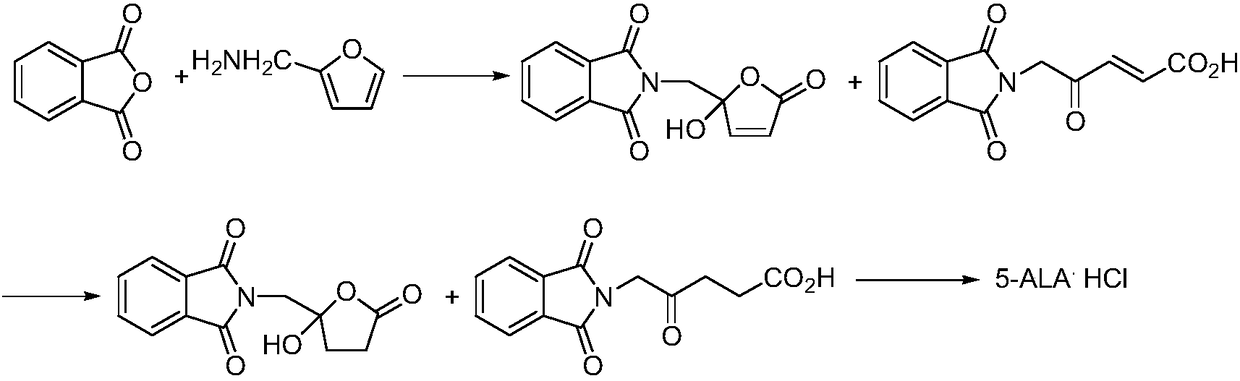
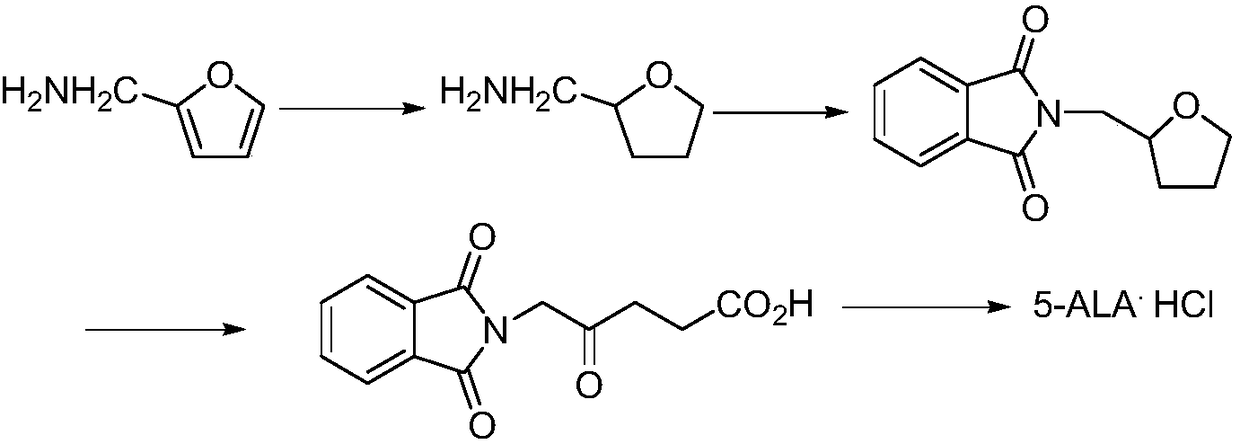
Preparation methods for 5-aminolevulinic acid hydrochloride and 5-aminolevulinic acid hydrochloride intermediate
A technology of aminolevulinic acid hydrochloride and intermediates, which is applied in the field of preparation of photosensitizer 5-aminolevulinic acid hydrochloride and intermediates, and can solve the conditions of heavy pollution and operation in the hydrochloride preparation process Harsh, expensive raw materials and other problems, to achieve the effect of easy crystallization and purification, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]
[0077] In a 2000ml four-necked flask, add 1000ml of dichloromethane, 183g DMAP, 250g of DCC and 144g of isopropylidene malonate, and after stirring at room temperature for 15 minutes, dropwise add 132g of monomethyl succinate dissolved in 200ml of dichloromethane After the addition was completed, the solution was heated to reflux, concentrated after the reaction was detected by TLC, and the obtained solid was washed with water, dried in vacuum and directly used in the next step.
[0078] The product in the previous step was dissolved in 1000ml of absolute ethanol, heated to reflux, concentrated after the TLC detection reaction, and 170g of the product obtained was directly used in the next step. (two-step yield>90%, purity>97%) 1 H NMR (300M, CDCl 3 )δ: 1.26(t, 3H), 1.30(t, 3H), 2.65(t, 2H), 2.90(t, 2H), 3.51(s, 2H), 4.15(q, 2H), 4.20(q, 2H) .
[0079] Dissolve the product in the previous step in 1000ml of acetic acid, cool to 0°C, add dropwise a solution of 69g...
Embodiment 2
[0084]
[0085] 500 liters of reaction kettle, add 250 liters of dichloromethane, add 18.3kg DMAP, 25kg DCC and 14.4kg of isopropylidene malonate, stir at room temperature for 15 minutes, dropwise add 14.6kg of monoethyl succinate to dissolve in After the addition of 50 liters of dichloromethane solution was completed, it was heated to reflux, concentrated after the reaction was detected by TLC, and the obtained solid was washed with water, dried in vacuo and directly used in the next step.
[0086] The product in the previous step was dissolved in 200 liters of anhydrous methanol, heated to reflux, concentrated after the TLC detection reaction, and 17 kg of the obtained product was directly used in the next step. (two-step yield>90%, purity>97%) 1 H NMR (300M, CDCl 3 )δ: 2.65(t, 2H), 2.90(t, 2H), 3.51(s, 2H), 3.68(s, 2H), 3.78(s, 2H).
[0087] Dissolve the product in the previous step in 100 liters of acetic acid, cool to 0 degrees, add dropwise a solution of 6.9 kg of s...
Embodiment 3
[0093]
[0094] In a 2000ml four-neck flask, add 1000ml of dichloromethane, 183g DMAP, 250g of DCC and 144g of isopropylidene malonate, and after stirring at room temperature for 15 minutes, dropwise add 160g of monopropyl succinate dissolved in 200ml of dichloromethane After the addition was completed, the solution was heated to reflux, concentrated after the reaction was detected by TLC, and the obtained solid was washed with water, dried in vacuum and directly used in the next step.
[0095] The product in the previous step was dissolved in 1000ml of absolute ethanol, heated to reflux, concentrated after the TLC detection reaction, and 200g of the product obtained was directly used in the next step (two-step yield>90%, purity>97%). 1 H NMR (300M, CDCl 3 )δ: 1.26(t, 3H), 1.30(t, 3H), 2.65(t, 2H), 2.90(t, 2H), 3.51(s, 2H), 4.15(q, 2H), 4.20(q, 2H) .
[0096] Dissolve the product in the previous step in 1000ml of acetic acid, cool to 0°C, add dropwise a solution of 69g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com