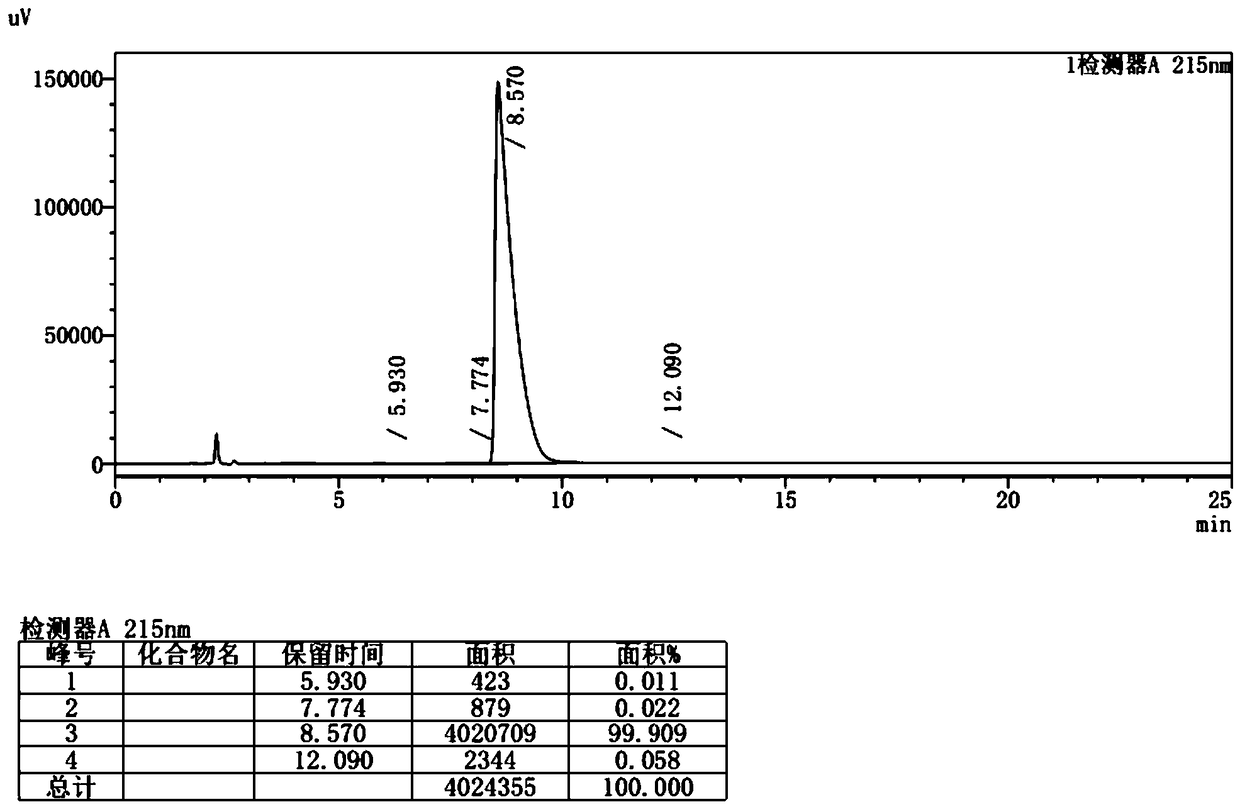
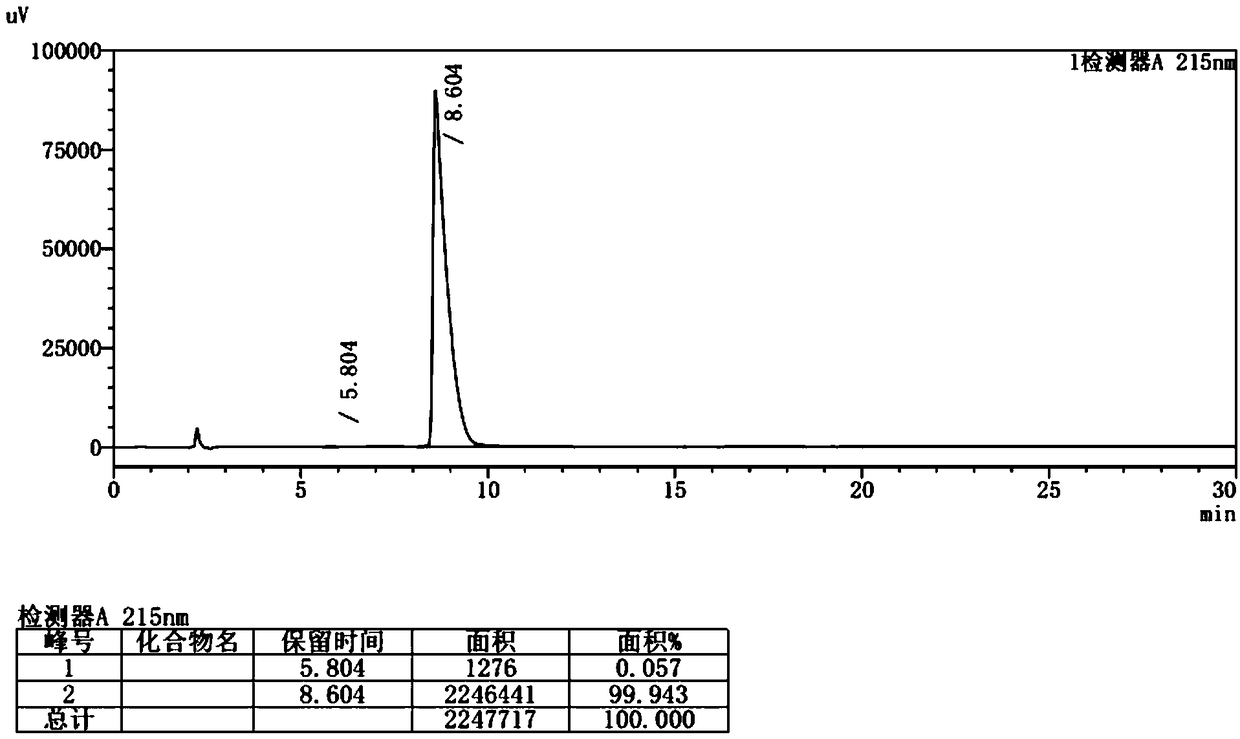
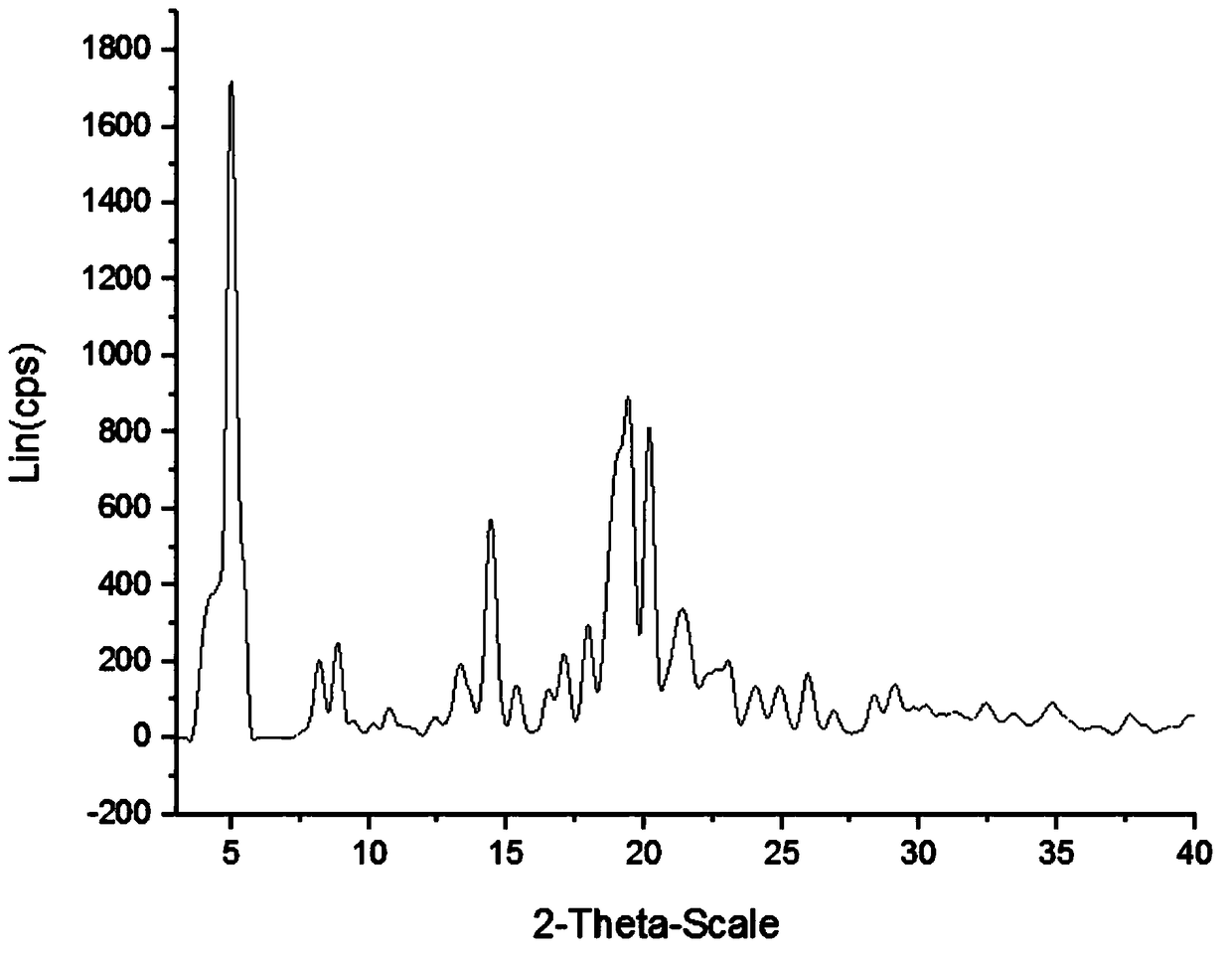
Method for safely preparing pimavanserin and tartrate thereof by utilizing triphosgene
A technology of pimavanserin and tartrate, which is applied in the field of synthesis of pharmaceutical molecules, can solve the problems of great impact on operator's health, expensive DPPA, unfavorable to industrialized production, etc., so as to reduce production safety risks and avoid Raney nickel. easy-to-use, easy-to-achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of compound 3 from compound 1
[0079]
[0080] 1) Add p-hydroxybenzaldehyde (14.6g, 122.8mmo1), anhydrous potassium carbonate (25.4g, 184.2mmoL), potassium iodide (1.4g, 12.3mmoL) and 75mL DMF into a 250mL three-necked flask, and heat to 85°C;
[0081] 2) Slowly add chloroisobutane (34.1g, 368.4mmol) to the reaction system, after the addition is complete, the system reacts at 85°C for 6h;
[0082] 3) Stop the reaction, cool the system to room temperature, filter, and rinse the filter cake twice with 90 mL of ethyl acetate;
[0083] 4) Pour the filtrate into 250 mL of water, separate the organic phase, extract the water phase three times with 90 mL of ethyl acetate, combine the organic phases, wash once with 150 mL of saturated brine, dry over anhydrous sodium sulfate, filter, and spin the filtrate to obtain 19.4 g Compound, light yellow liquid, yield 90%.
[0084] MS (m / z): [M+H] + =176.2; The NMR data of compound 3 are as follows: 'H NMR (400MHz, C...
Embodiment 2
[0100] Preparation of compound 3 from compound 1
[0101]
[0102]1) Add p-hydroxybenzaldehyde (14.6g, 122.8mmo1), anhydrous potassium carbonate (25.4g, 184.2mmoL), potassium iodide (1.4g, 12.3mmoL) and 75mL DMF into a 250mL three-necked flask, and heat to 90°C;
[0103] 2) Slowly add bromoisobutane (50.5g, 368.4mmol) into the reaction system, after the addition is complete, the system reacts at 90°C for 5h;
[0104] 3) Stop the reaction, cool the system to room temperature, filter, and rinse the filter cake twice with 90 mL of ethyl acetate;
[0105] 4) Pour the filtrate into 250 mL of water, separate the organic phase, extract the water phase three times with 90 mL of ethyl acetate, combine the organic phases, wash once with 150 mL of saturated brine, dry over anhydrous sodium sulfate, filter, and spin the filtrate to obtain 19.8 g Compound, light yellow liquid, yield 92%.
[0106] Preparation of compound 4 from compound 3
[0107]
[0108] 5) Add 4-isobutoxyben...
Embodiment 3
[0118] Preparation of compound 3 from compound 1
[0119]
[0120] 1) Add p-hydroxybenzaldehyde (14.6g, 122.8mmo1), anhydrous potassium carbonate (25.4g, 184.2mmoL), potassium iodide (1.4g, 12.3mmoL) and 75mL DMF into a 250mL three-necked flask, and heat to 100°C;
[0121] 2) Slowly add iodoisobutane (67.8g, 368.4mmol) into the reaction system, after the addition is complete, the system reacts at 100°C for 5h;
[0122] 3) Stop the reaction, cool the system to room temperature, filter, and rinse the filter cake twice with 90 mL of ethyl acetate;
[0123] 4) Pour the filtrate into 250 mL of water, separate the organic phase, extract the water phase three times with 90 mL of ethyl acetate, combine the organic phases, wash once with 150 mL of saturated brine, dry over anhydrous sodium sulfate, filter, and spin the filtrate to obtain 19.6 g Compound, light yellow liquid, yield 91%.
[0124] Preparation of compound 4 from compound 3
[0125]
[0126] 5) Add 4-isobutoxyb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com