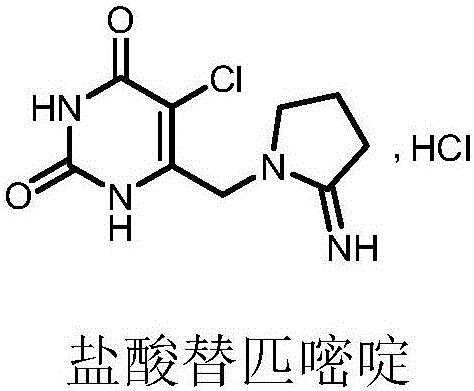
Preparation method of tipiracil
A technology for tipiramidine and pyrimidinedione is applied in the field of preparation of tipiramidine, which can solve the problems of low yield and poor purity, and achieve the effects of high yield, improved selectivity and reduced risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Dissolve 10.0g of 6-chloromethyluracil in 100ml of dimethyl sulfoxide, add 17.0g of chlorosuccinimide with stirring at room temperature, raise the temperature to 60°C after the addition, and carry out the heat preservation reaction for 4h . The reacted system was added to 400ml of water to precipitate a light yellow solid. After suction filtration and drying, 11.0g of intermediate A was obtained, with a yield of 90.7%.
[0033] 2) Add 33.6ml of n-butyllithium n-hexane solution (2mol / L) into the reaction flask, add 50ml of tetrahydrofuran, slowly add 5.9g of α-pyrrolidone into the reaction flask, and control the temperature at 0-5°C for reaction. After completion, add dropwise to 9.0g of intermediate A in 50ml of tetrahydrofuran solution, then control the temperature to 5°C for 3 hours of reaction, after the reaction is completed, warm up to room temperature, filter with suction, add water to beat the solid, wash with water, filter with suction, and dry to obtain 10.2...
Embodiment 2
[0036] 1) Dissolve 10.0g of 6-chloromethyluracil in 100ml of N,N-dimethylformamide, add 16.5g of chlorosuccinimide with stirring at room temperature, heat up to 60°C after addition, and Insulation reaction was carried out for 3h. The reacted system was added to 400ml of water to precipitate a light yellow solid. After suction filtration and drying, 11.4g of intermediate A was obtained, with a yield of 94.0%.
[0037] 2) Add 37.5ml of lithium diisopropylamide THF solution (2.0mol / L) into the reaction flask, add 50ml of methanol, slowly drop 6.6g of α-pyrrolidone into the reaction flask, and control the temperature at 0-5°C for reaction After the reaction is complete, add it dropwise to 10 g of intermediate A in methanol solution, then control the temperature to 0 ° C for 3 hours, after the reaction is completed, warm up to room temperature, filter with suction, add water to beat, wash with water, filter with suction, and dry to obtain 11.3 g Intermediate B, yield 90.0%.
[00...
Embodiment 3
[0040] 1) Dissolve 10.0g of 6-chloromethyluracil in 100ml of N,N-dimethylformamide, add 16.8g of chlorosuccinimide with stirring at room temperature, raise the temperature to 60°C after the addition, and carry out Insulation reaction 4h. The reacted system was added to 400ml of water to precipitate a light yellow solid. After suction filtration and drying, 11.2g of intermediate A was obtained, with a yield of 92.3%.
[0041] 2) Add 38.2ml of n-butyllithium n-hexane solution (2mol / L) into the reaction flask, add 50ml of methanol, slowly drop 6.5g of α-pyrrolidone into the reaction flask, and control the temperature at 0-5°C for reaction. After completion, add dropwise to 10g of intermediate A in methanol solution, then control the temperature to 0°C for 2 hours of reaction, after the reaction is completed, warm up to room temperature, filter with suction, add water to beat, wash with water, filter with suction, and dry to obtain 11.2g of intermediate B , yield 89.2%.
[0042]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com