Preparation method of polyalcohol acetal compound
A technology of alcohol acetal and polyol, which is applied in the field of preparation of polyol acetal compounds, can solve the problems of large influence of type and structure, easy production of by-products, poor product selectivity, etc., and achieve wide applicability and route design Novelty and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1,3:2,4-di-o-hydroxyphenylmethylene-D-sorbitol, obtained by condensation of sorbitol and salicylaldehyde, its synthetic route is as follows:
[0029]
[0030] Its preparation method comprises the following steps:
[0031] In a 100mL four-necked flask, add 9.11g (0.05mol) of sorbitol, 10ml of concentrated hydrochloric acid, 0.5g of phosphotungstic acid, 10ml of trimethyl orthoformate, 40ml of DMSO, and stir for 10min, then add 12.1g (0.10mol) of salicylaldehyde ), and react at a temperature of 20-30°C for 12h. Add 30ml of water, stir for 30min, and filter with suction to obtain a solid. The product was washed twice with 50 ml of water, then twice with ice ethanol, and dried to constant weight. The obtained product was 14.4g, yield: 73.8%, melting point: 206.5-207.8°C.
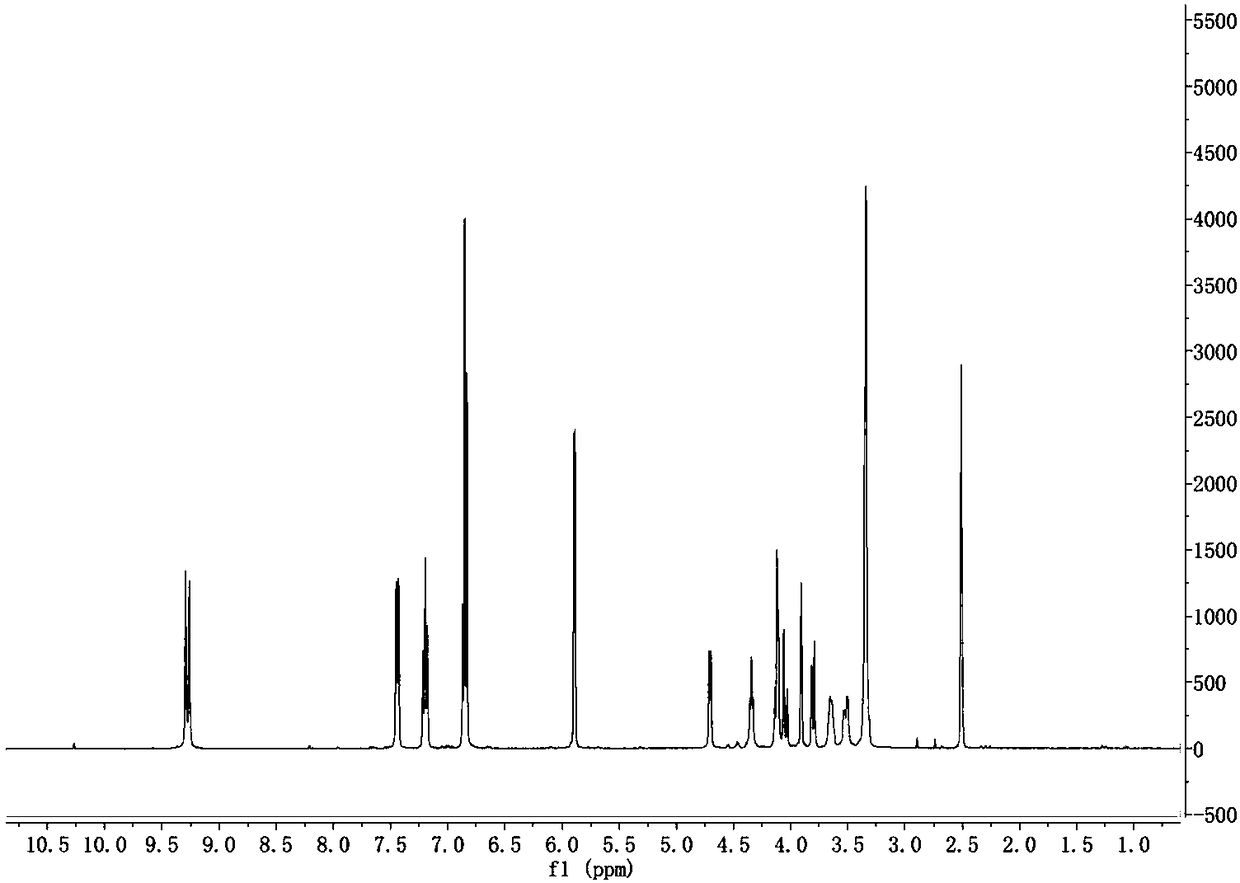
[0032] 1H NMR (400MHz, DMSO-d 6, TMS, 25℃): δ9.20.-9.23(d, J=12.8Hz, 2H, Ar-OH), 7.37-7.39(d, J=7.7Hz, 2H, Ar-H), 6.78-6.80( d,J=7.5Hz,2H,Ar-H),7.46-7.51(s,1H,Ar-H),5.82-5.85(d,J=3.3Hz,2H,OCHO),4....
Embodiment 2
[0037] 1,3:2,4-Di-n-octyl-D-sorbitol, obtained by condensation of sorbitol and n-octylaldehyde, its synthetic route and preparation method are similar to the synthetic route provided in Example 1, see Examples 1, which will not be elaborated here.
[0038] The yield of the resulting product: 82%, melting point: 112.5-114.7°C
[0039] 1H NMR (400MHz, DMSO-d 6 ,TMS,25℃):4.40-4.55(m,2H,OCHO),3.52-3.60(m,8H,OH),3.50-3.31(m,8H,CH)1.58-1.43(m,2H,CH 2 ), 1.40-1.12 (m, 24, CH 2 ),0.83-0.75(t,J=6.7Hz 6H,CH 3 ).
[0040] Two, the synthesis of mannitol bicondensate (compound 6)
[0041] The synthetic route is shown in formula (II):
[0042]
[0043] Wherein, compound 4 is mannitol; Ar 1 To replace the aliphatic aldehyde moiety, compound 5 is an aliphatic aldehyde.
Embodiment 3
[0045] 1,3:2,4-Di-n-octyl-mannitol, obtained by condensation of mannitol and n-octylaldehyde, its synthetic route is shown in formula (II), and its preparation method is similar to the synthetic route provided in Example 1 , can refer to Embodiment 1, and will not be described in detail here.
[0046] Yield of the product obtained: 72%, melting point: 109.3-111.2°C.
[0047] 1H NMR (400MHz, DMSO-d 6 ,TMS,25℃):4.75-4.90(m,2H,OCHO),4.05-3.92(m,2H,OH),3.90-3.31(m,8H,CH)1.56-1.41(m,2H,CH 2 ), 1.40-1.12 (m, 24, CH 2 ),0.82-0.73(t,J=6.7Hz 6H,CH 3 ).
[0048] Three, the synthesis of mannitol tricondensate (compound 8)
[0049] The synthetic route is as shown in formula (Ⅲ):
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com