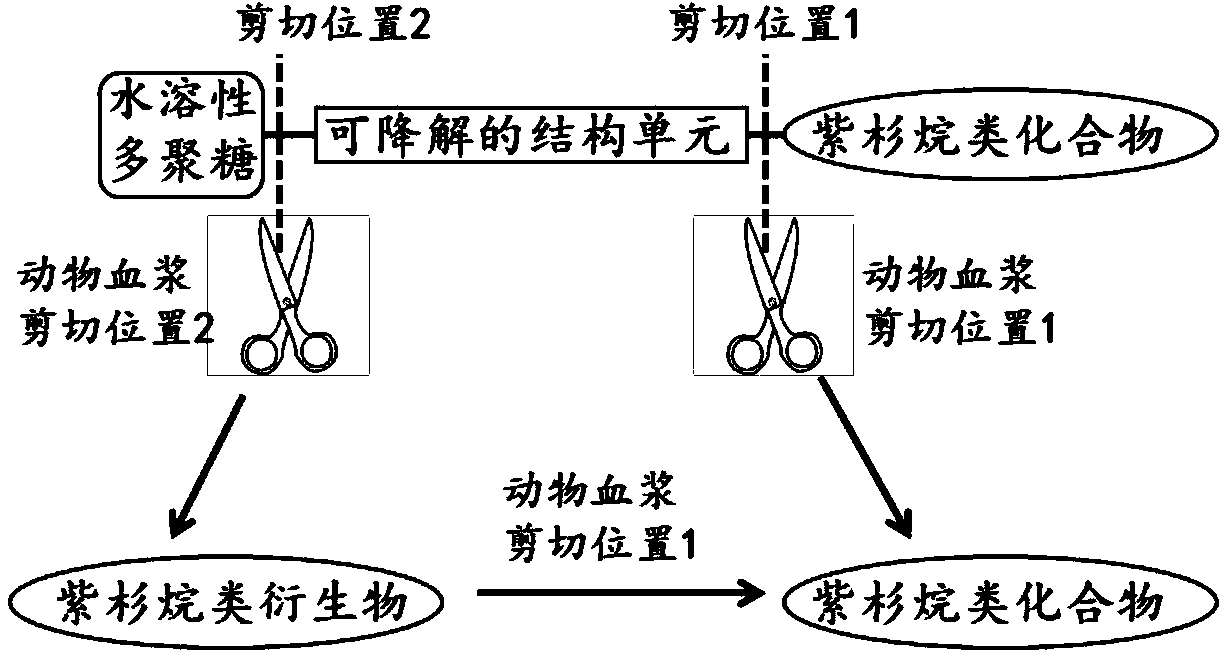
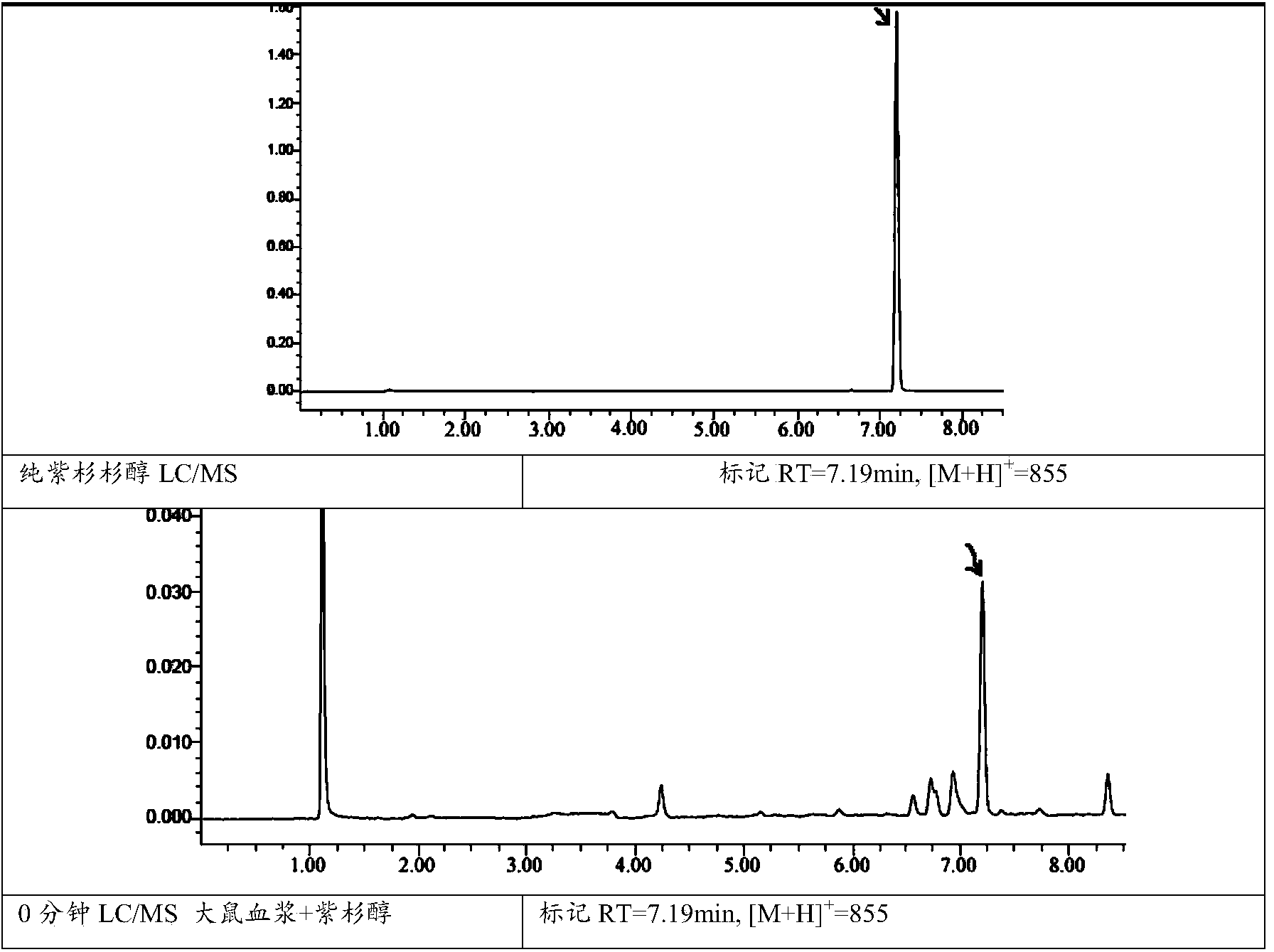
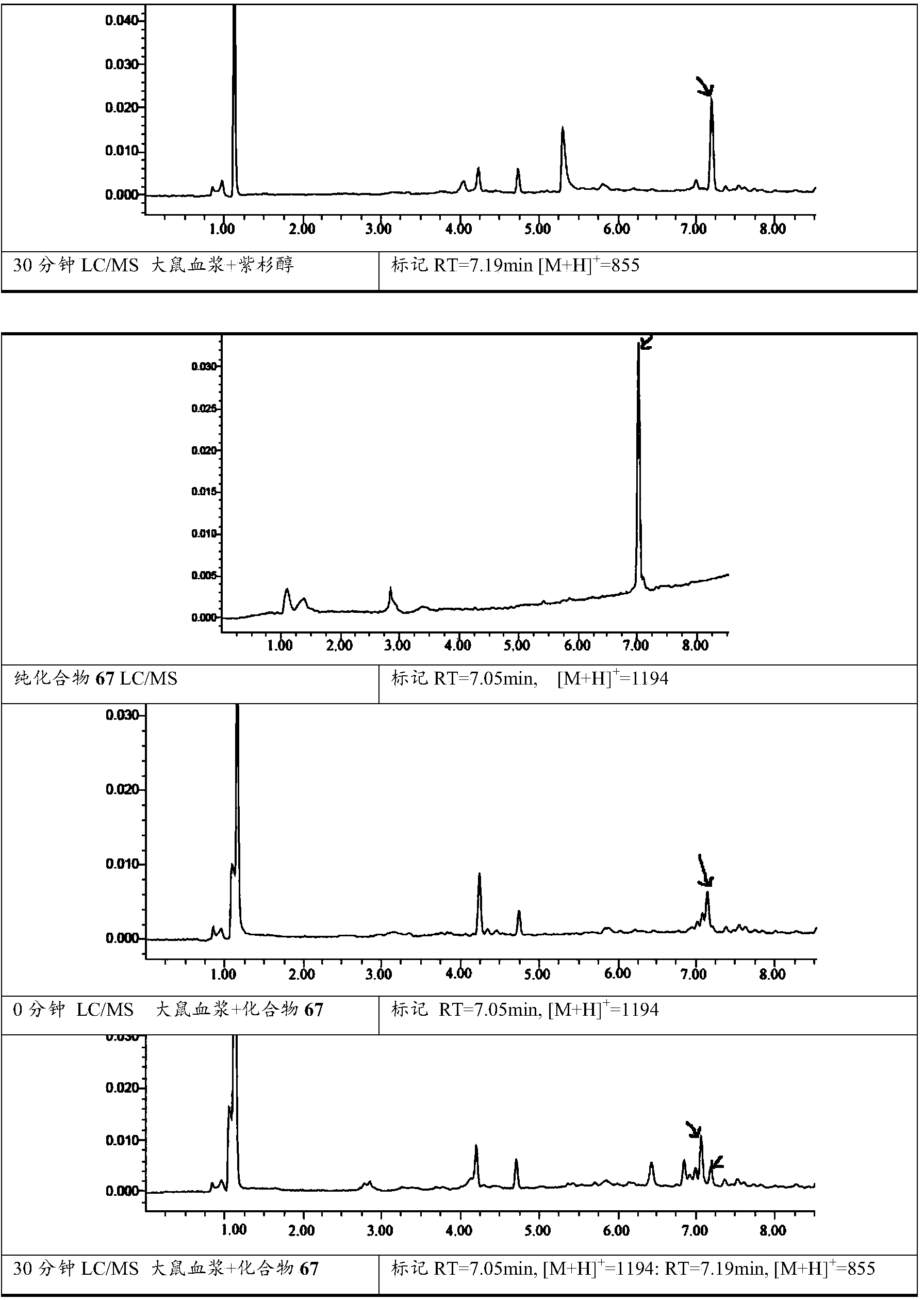
Covalent polycompound of water-soluble polysaccharide and taxane compound, and preparation method and medical application of covalent polycompound
A water-soluble polysaccharide technology, which is applied in chemical instruments and methods, active ingredients of silicon compounds, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of difficult covalents and low yields, etc. Achieve the effect of good water solubility and single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0231] Embodiment 1: the synthesis of compound 1
[0232] Into a 250mL round bottom flask, add 9.32g (11.53mmol) of docetaxel, 2.20g (33.84mmol) of imidazole and 20mL of dimethylformamide solvent, and then add 5.21g (34.59mmol) of tert-butyldimethyl chloride silane. Stir for 12 hours at room temperature. After the reaction was complete, the reaction mixture was partitioned between saturated sodium chloride solution (250 mL) and ethyl acetate (250 mL), and the ethyl acetate layer was separated. The ethyl acetate layer was further washed twice with saturated sodium chloride solution (250mL×2) and dried over anhydrous magnesium sulfate, filtered, spin-dried, and subjected to silica gel column chromatography (eluent: ethyl acetate: petroleum ether / 10-70 %) to obtain 9.89g compound 1. Yield: 93%.
[0233] NMR analysis: 1 H NMR (300MHz, DMSO-D 6):δ7.98(d,2H,J=7.2Hz),7.72(t,1H,J=7.2Hz),7.63(t,2H,J=7.2Hz),7.58(d,1H,J=9.0Hz ),7.37(m,4H),7.15(brs,1H),5.77(t,1H,J=9.0Hz),5.39(d,1H,...
Embodiment 2
[0236] Embodiment 2: the synthesis of compound 2
[0237] Under the protection of nitrogen, 3.00g (3.25mmol) of compound 1 and 30mL of anhydrous tetrahydrofuran solvent were successively added to a 100mL round bottom flask, cooled to -70°C, and then 7.15mL (7.15mmol) of bis(trimethylsilyl)amino Lithium tetrahydrofuran solution, stirred for 1 hour. Then, 1.22 g of benzyl chloroformate (7.15 mmol) were added and the coolant was removed. After 2 hours, the reaction naturally rose to room temperature, at which point 3.0 mL of acetic acid was added to stop the reaction. After spinning to dryness, the reaction mixture was partitioned between saturated sodium chloride solution (100 mL) and ethyl acetate (100 mL), and the ethyl acetate layer was separated. The ethyl acetate layer was further washed twice with saturated sodium chloride solution (100mL×2) and dried over anhydrous magnesium sulfate, filtered, spin-dried, and subjected to silica gel column chromatography (eluent: ethyl ...
Embodiment 3
[0241] Embodiment 3: the synthesis of compound 3
[0242] Into a 100 mL round-bottomed flask, 3.50 g of compound 2, 10 mL of tetrahydrofuran and tetrabutylammonium fluoride (1.0 mmol×5.88 mL) were sequentially added, and stirred at room temperature for 2 hours. After the reaction was completed, the product was spin-dried and subjected to silica gel column chromatography (eluent: ethyl acetate: petroleum ether / 10-50%) to obtain 2.82 g of compound 3. Yield: 96%.
[0243] NMR analysis: 1 H NMR (300MHz, CDCl 3 ):δ8.10(d,2H,J=7.2Hz),7.63(t,1H,J=7.2Hz),7.51(t,2H,J=7.2Hz),7.32–7.40(m,10H),6.28 (s,1H),6.20(brs,1H),5.70(d,1H,J=7.2Hz),5.54(t,1H,J=9.0Hz),5.42(d,1H,J=7.2Hz),5.29 (d,1H,J=7.2Hz),5.20(m,4H),4.95(d,1H,J=9.0Hz),4.66(s,1H),4.32(d,1H,J=9.0Hz),4.18 (d,1H,J=9.0Hz),3.93(d,1H,J=6.6Hz),3.41(s,1H),2.62(m,1H),2.40(s,3H),2.33(m,1H) ,2.10(s,3H),1.97(m,4H),1.84(s,3H),1.65(m,1H),1.43(m,1H),1.37(s,9H),1.24(s,3H), 1.17(s,3H);
[0244] 13 C NMR (125MHz, CDCl 3 ):δ201.5, 174.9, 172.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com