Synthesis method of 2, 9-substituted 4-halogenated-1, 10-phenanthroline
A technology of o-phenanthroline and its synthesis method, which is applied in the field of synthesis of 4-halogenated-1,10-phenanthroline, which can solve the problems of low conversion rate, difficulty in purification, unfavorable industrial production, etc., so as to improve the conversion rate , improve the yield, and benefit the effect of industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
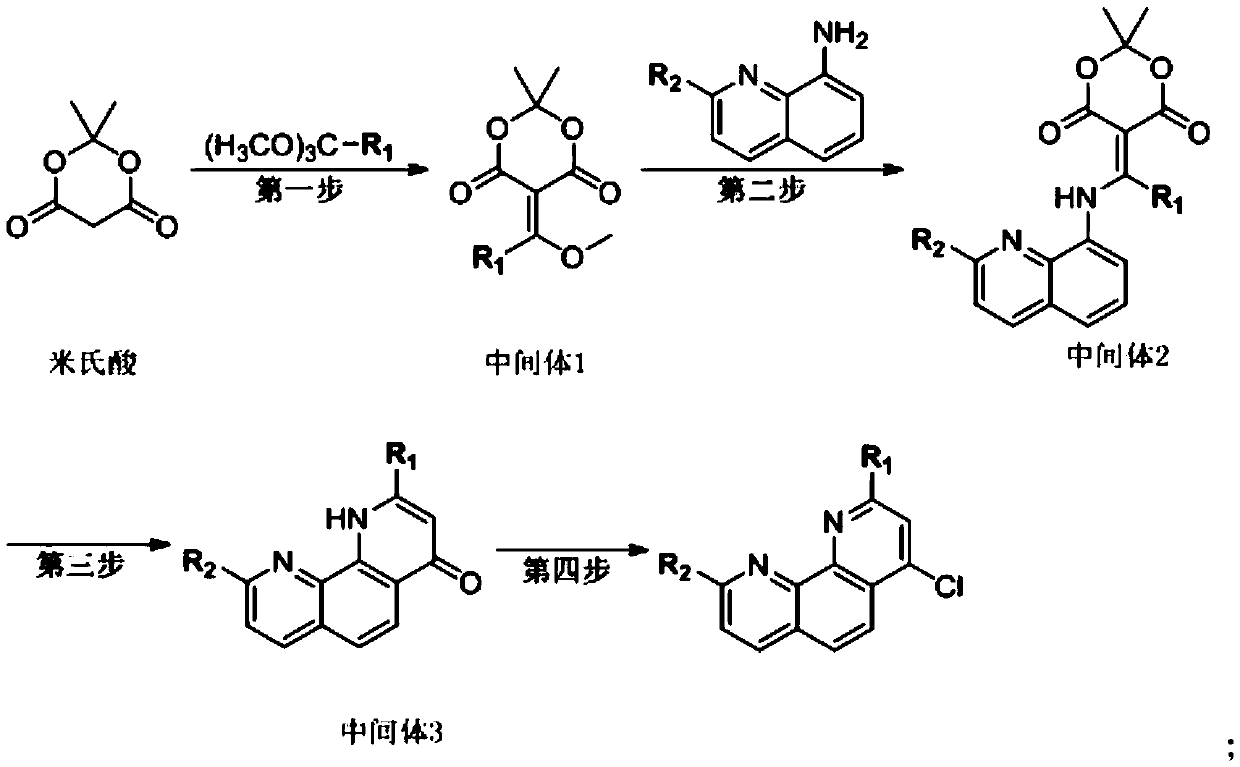
[0027] A kind of synthetic method of 4-chloro-2,9-dimethyl-1,10-phenanthroline, comprising the following steps:
[0028] first step:
[0029]
[0030] Under argon protection, add 273.41 g of trimethyl orthoacetate and 163.99 g of Michaelis acid to a 1L three-necked flask equipped with a mechanical stirrer, a thermometer, a condenser tube, a water separator, and a drying tube, start stirring and heating, and heat up (0.5 h) After reflux reaction at 78°C for 2 hours (75ml of low-boiling point by-products are fractionated while reacting), turn off the heating and cool down to 55°C for use;
[0031] Step two:
[0032]
[0033] Add 60.00 g of 2-methyl-8-aminoquinoline to the reaction solution in the first step, control the temperature at 60°C for 3 hours to stop the reaction, cool the reaction solution to 35°C for 30 minutes, and then filter to obtain a filter cake The crude yellow solid was beaten with 120.00g trimethyl orthoacetate at 35°C for 10min, filtered, and the fil...
Embodiment 2
[0041] A kind of synthetic method of 4-bromo-2,9-dimethyl-1,10-phenanthroline, comprising the following steps:
[0042] first step:
[0043]
[0044] Under argon protection, add 546.82 g of trimethyl orthoacetate and 163.99 g of Michaelis acid to a 1L three-necked flask equipped with a mechanical stirrer, a thermometer, a condenser tube, a water separator, and a drying tube, start stirring and heating, and heat up (0.5 h) After reflux reaction at 98°C for 4 hours (83ml of low-boiling point by-products are fractionated while reacting), turn off the heating and cool down to 65°C for use;
[0045] Step two:
[0046]
[0047]Add 30.00 g of 2-methyl-8-aminoquinoline to the reaction solution in the first step, and react at a temperature of 40°C for 5 hours to stop the reaction; cool the reaction solution to 25°C and keep stirring for 30 minutes, then filter to obtain a filter cake The crude yellow solid was beaten with 60.00g trimethyl orthoacetate at 25°C for 10min, filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com