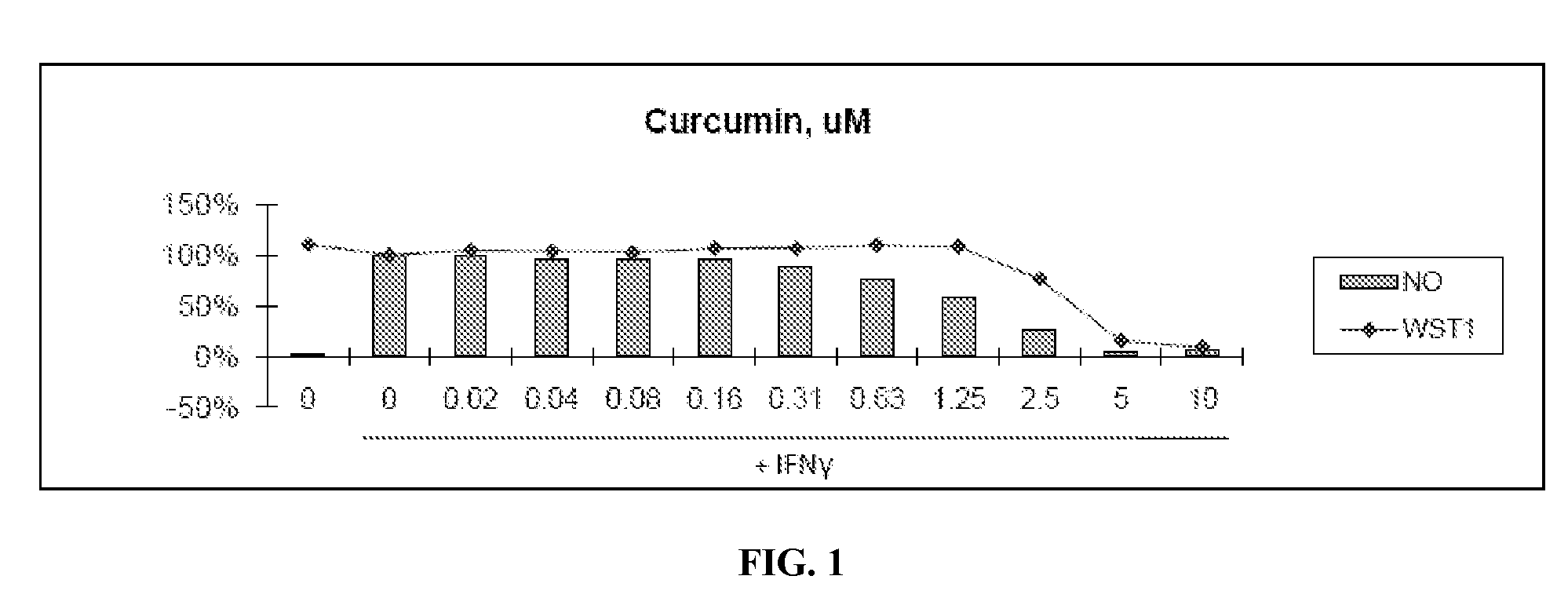
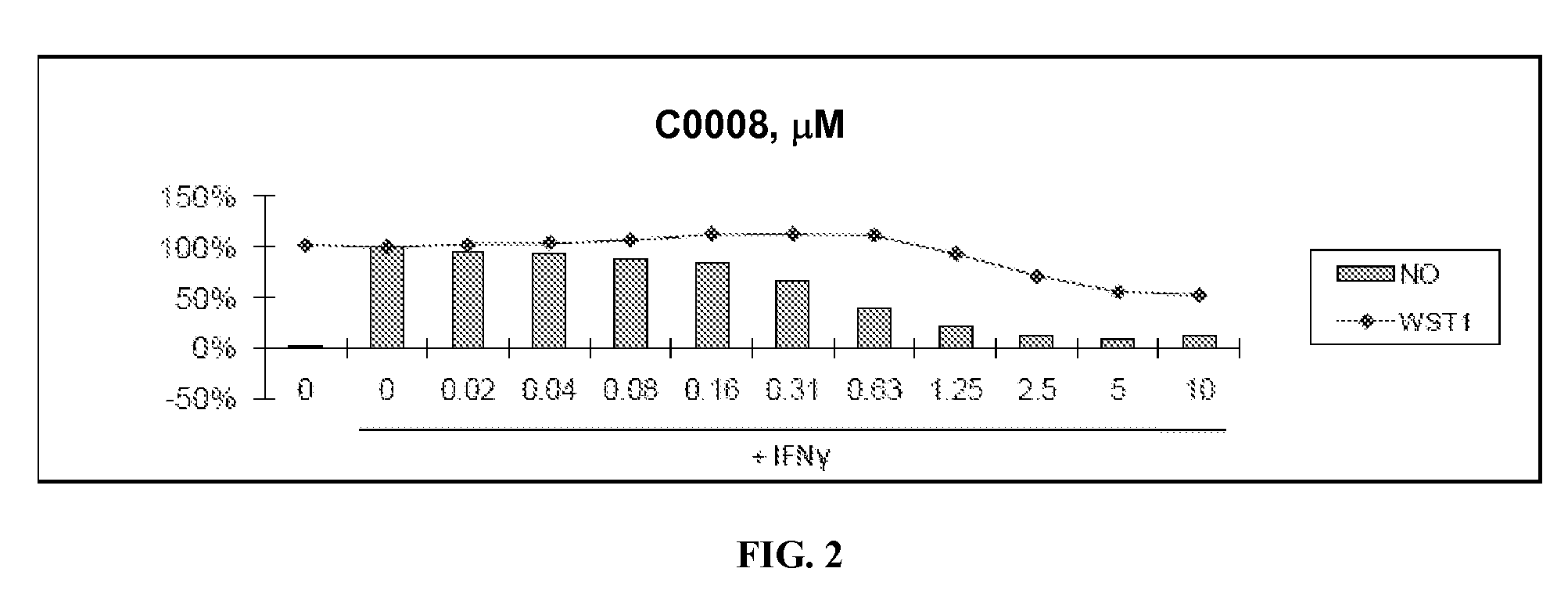
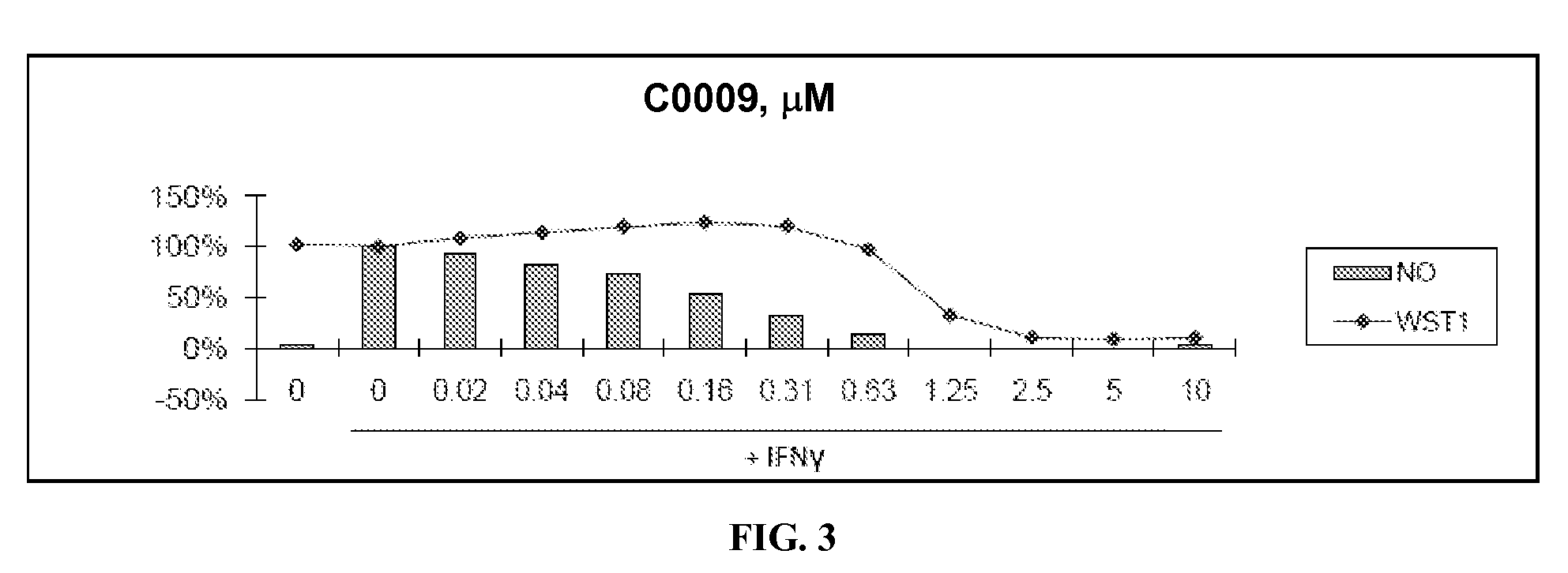
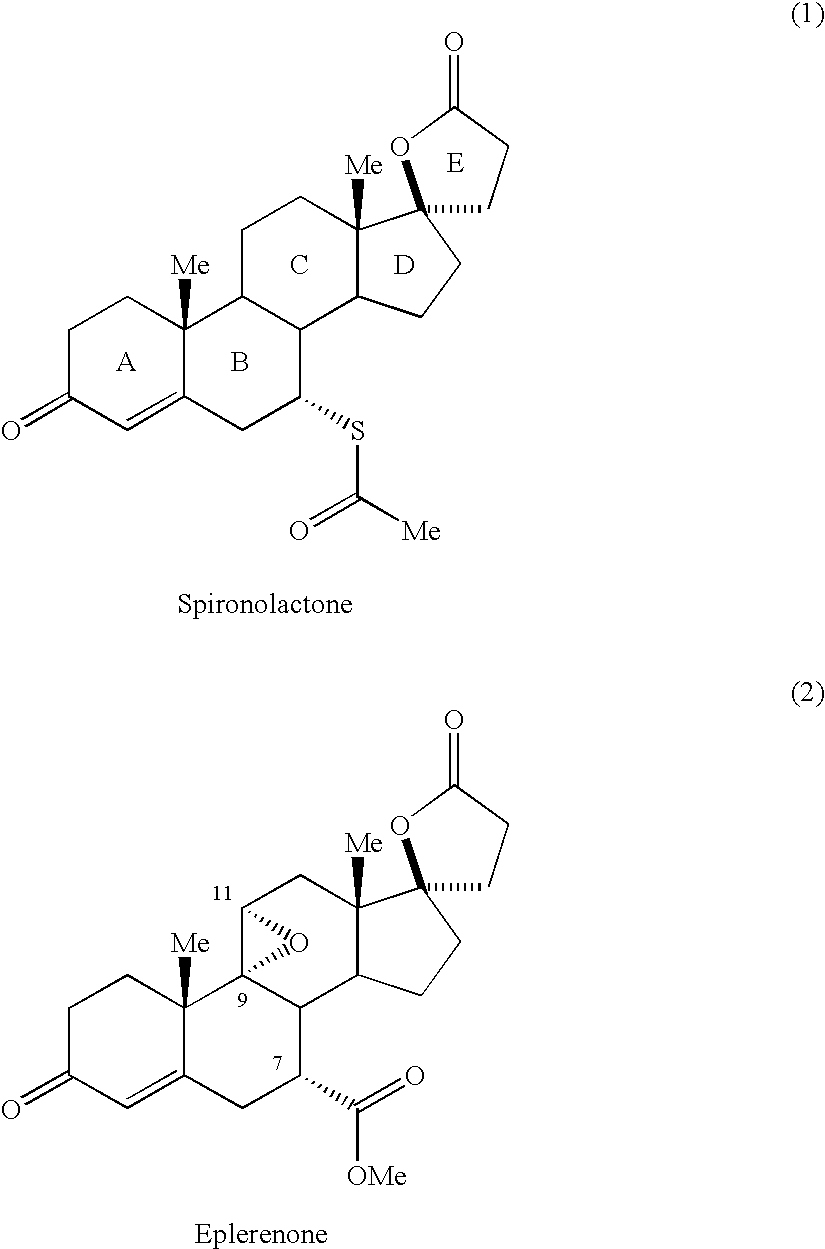
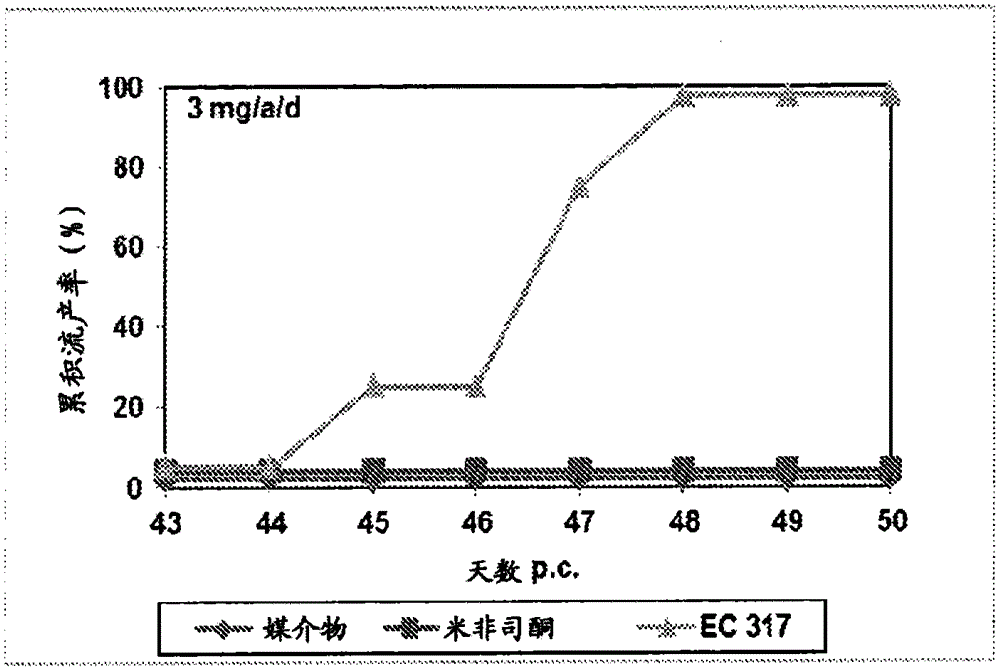
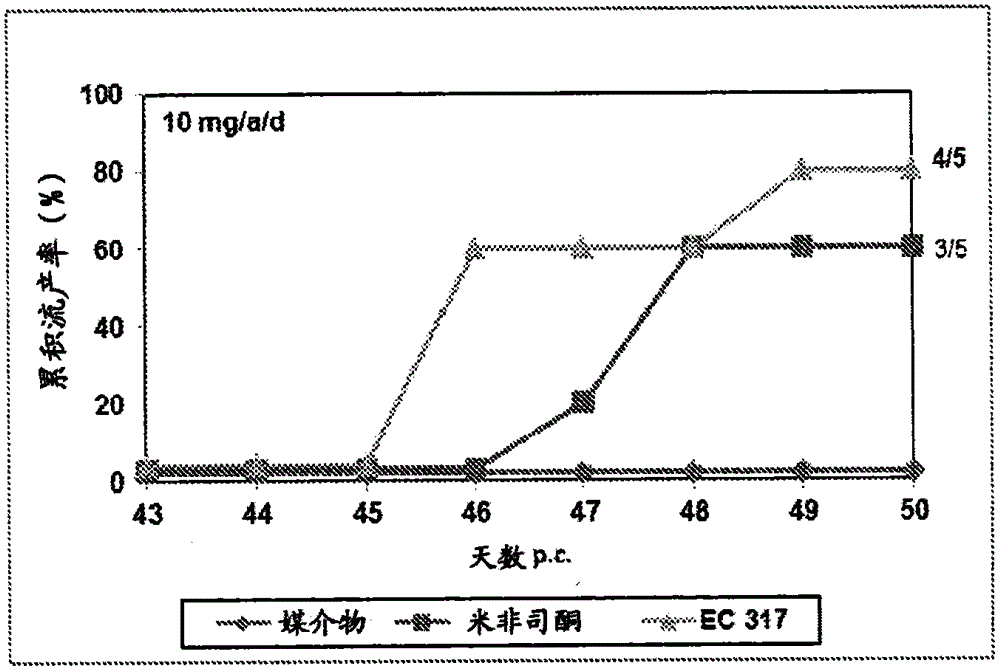
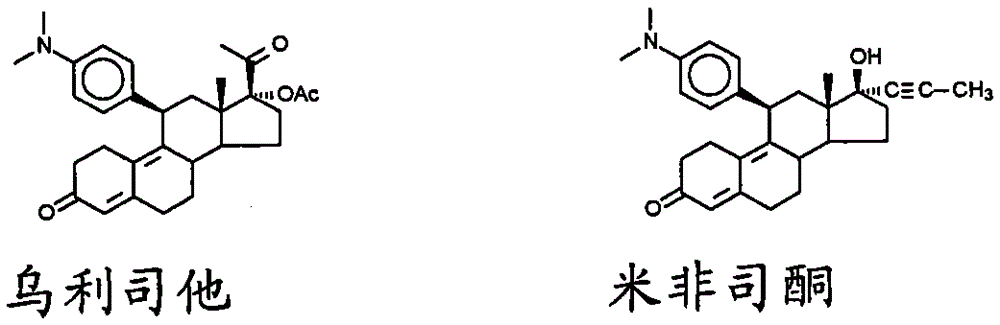
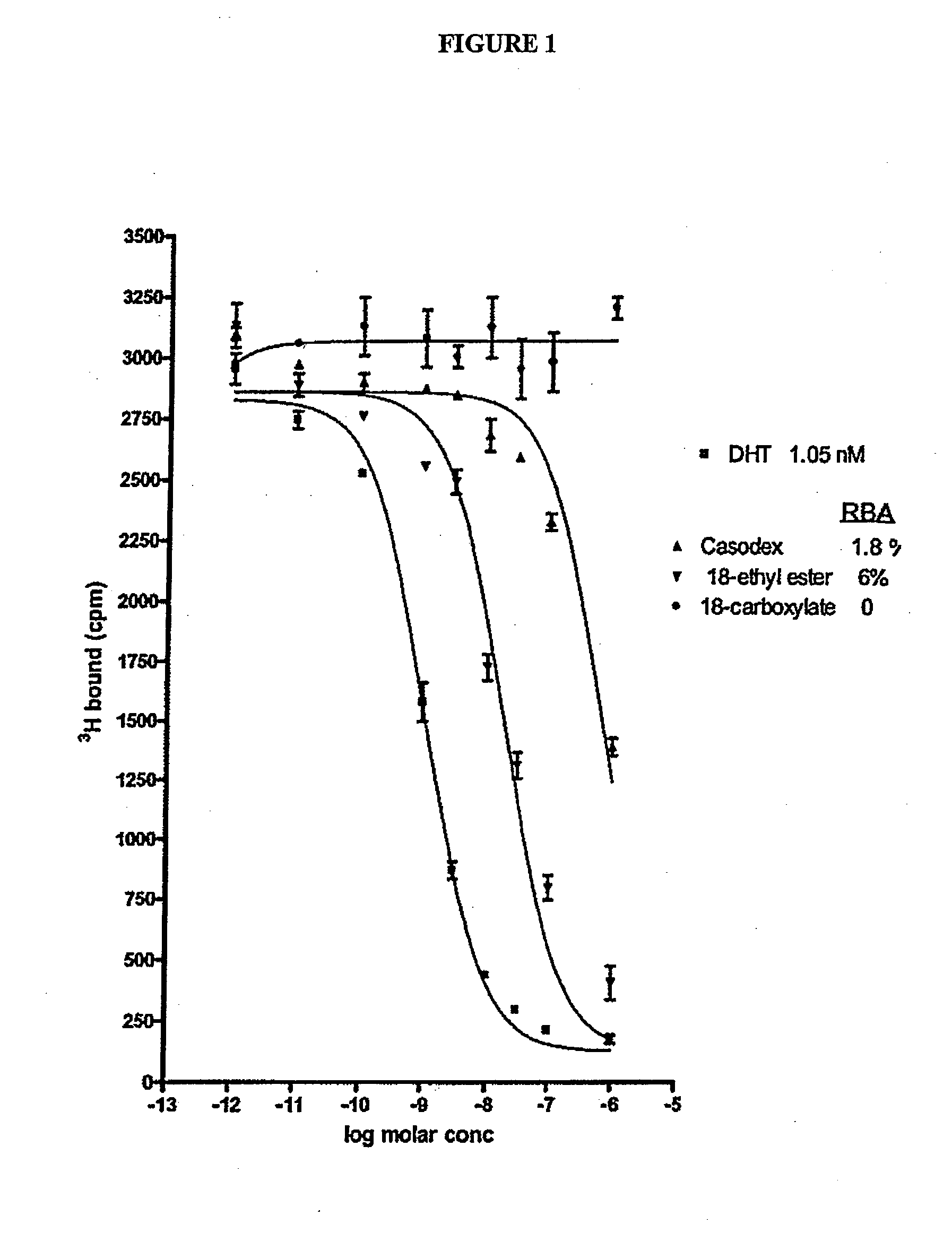
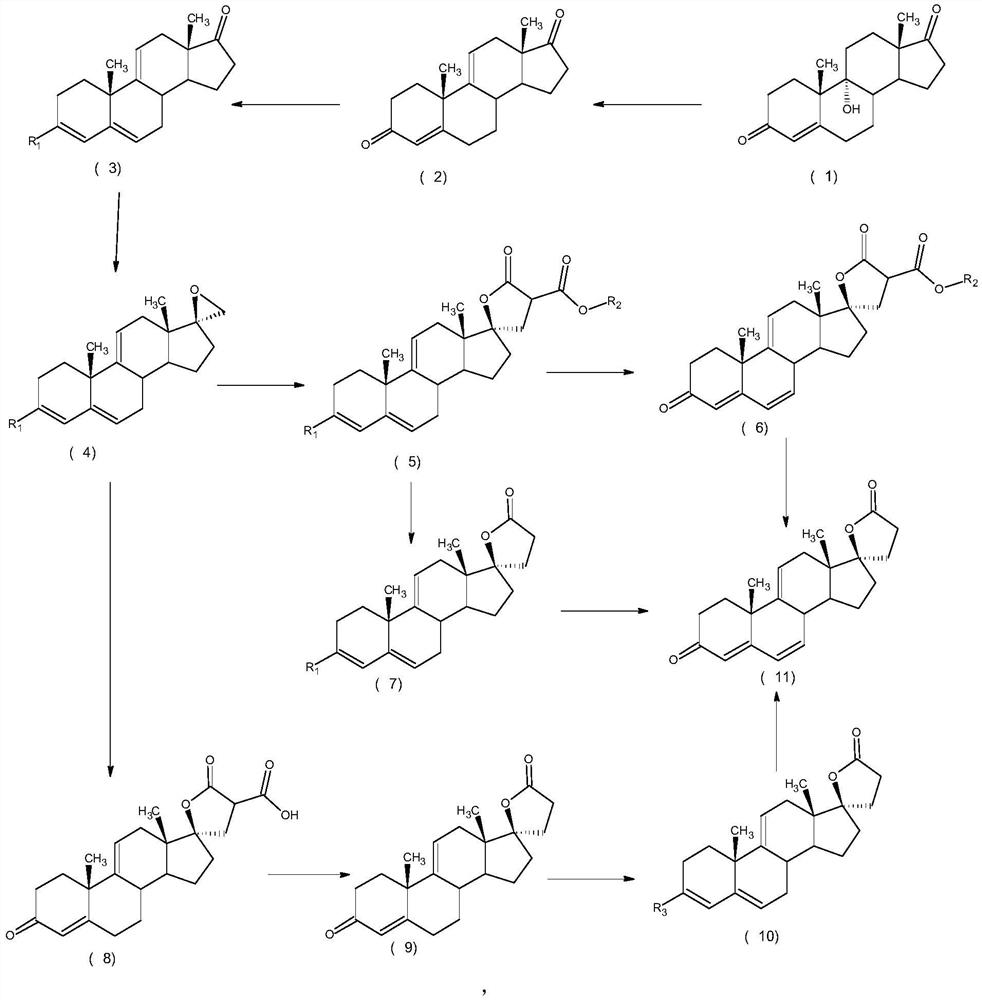
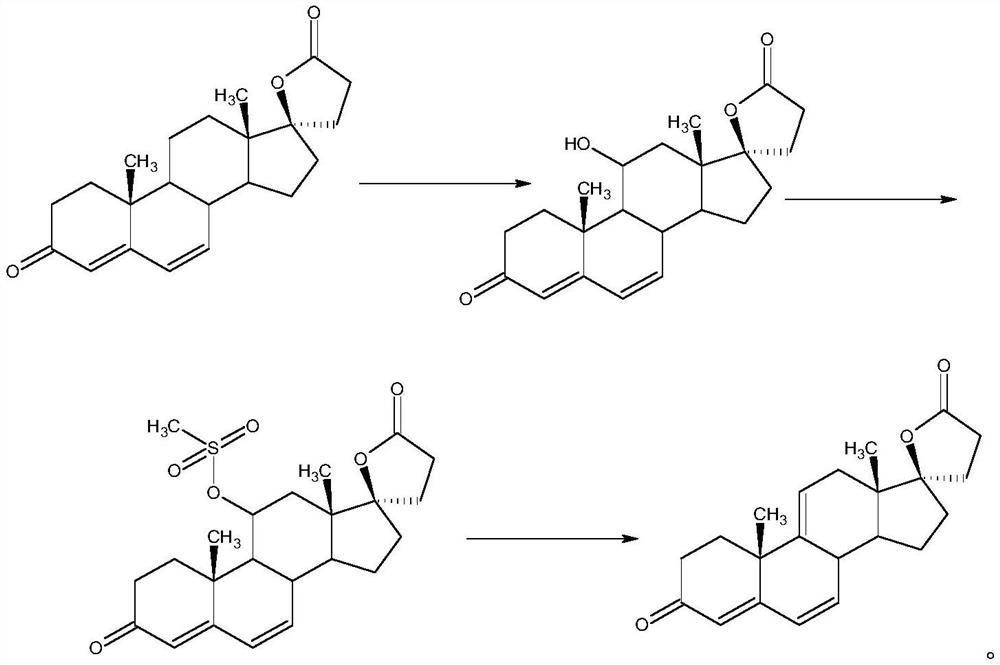
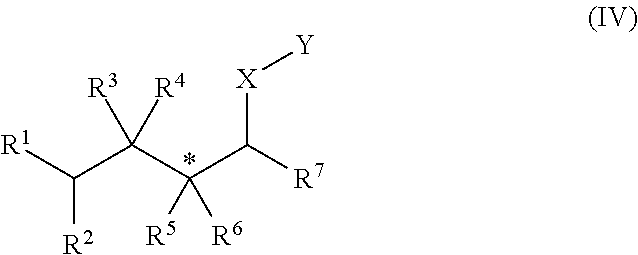
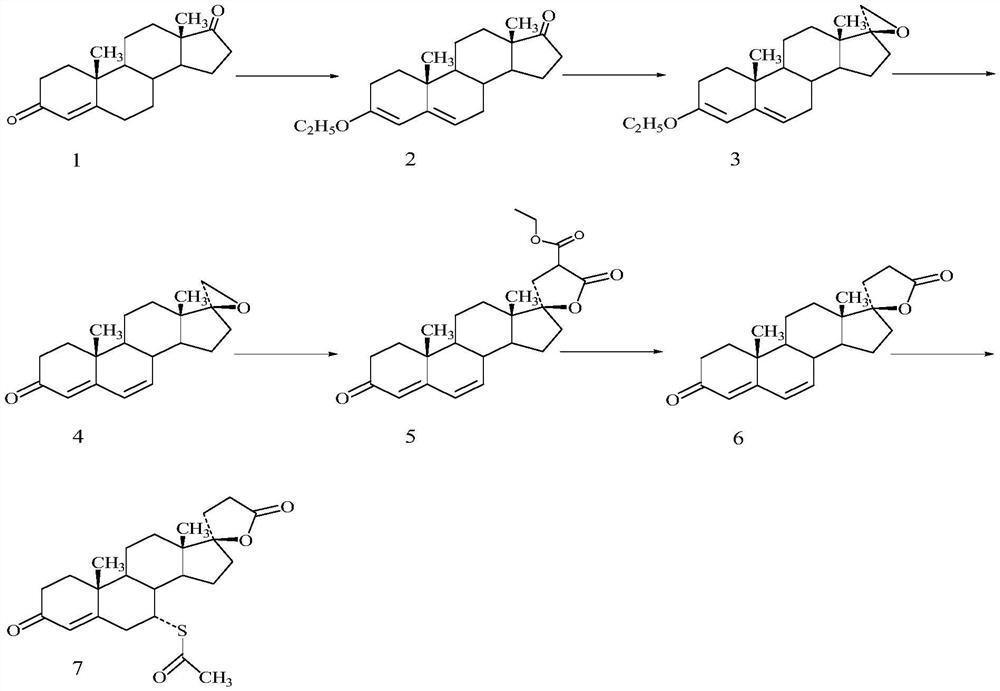
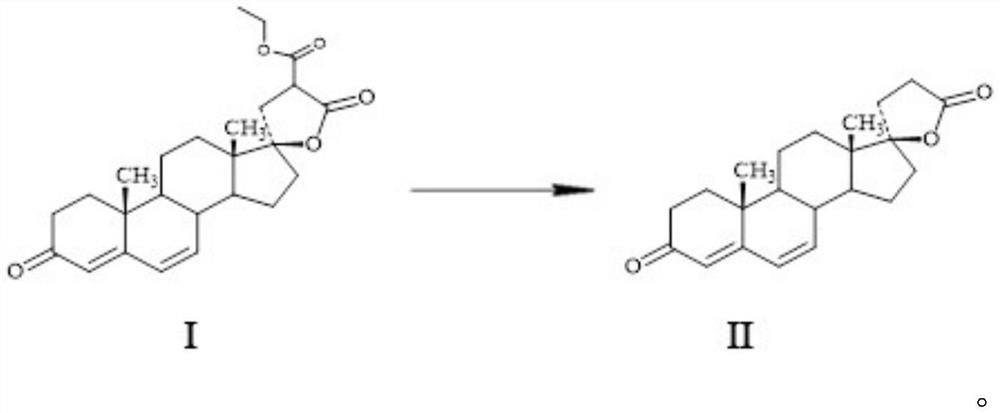
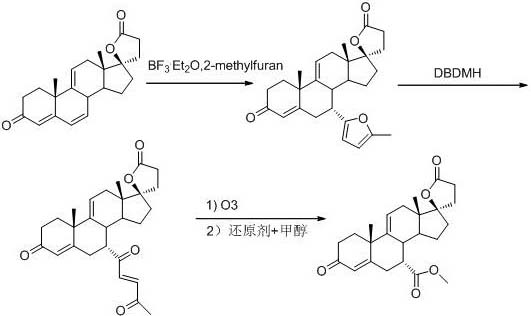
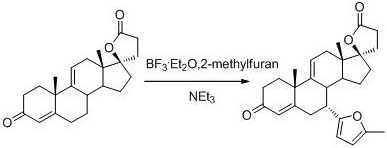
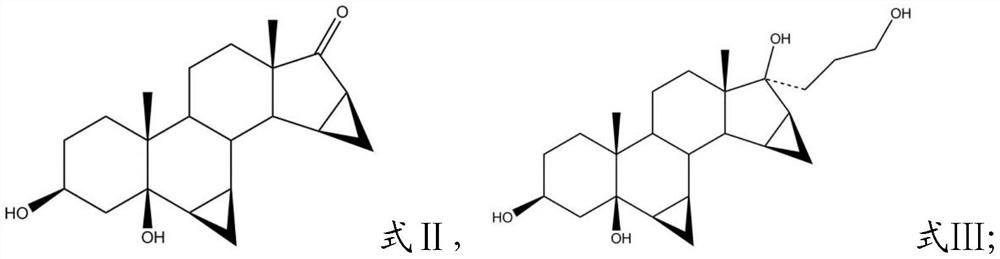
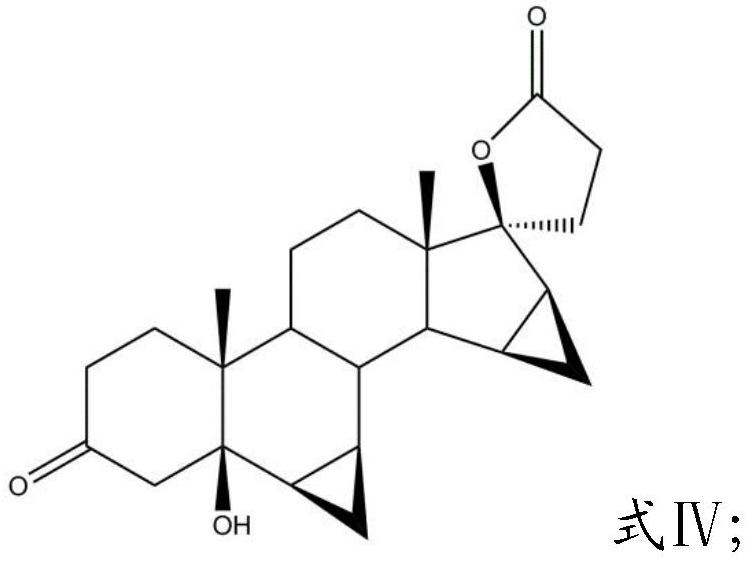
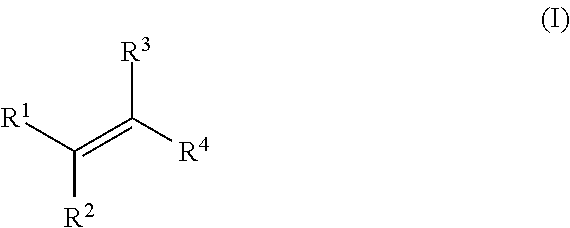Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48results about "Lactone steroids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dehydroandrosterone analogs including an anti-inflammatory pharmacore and methods of use
ActiveUS8258329B2Suppresses demyelinationSuppresses transectionSenses disorderNervous disorderStereochemistryAnti-inflammatory analgesics
Owner:REATA PHARMA INC
Novel PD-1 inhibitor and application thereof
PendingCN108727453AImprove bindingHigh binding affinityOrganic active ingredientsAntipyreticDiseasePharmacology
Owner:EAST CHINA UNIV OF SCI & TECH
Processes for preparation of 9,11-epoxy steroids and intermediates useful therein
InactiveUS6180780B1Improve securityFacilitating control of thermal profileOrganic active ingredientsMicrobiological testing/measurementCombinatorial chemistryEpoxymexrenone
Multiple reaction schemes, process steps and intermediates are provided for the synthesis of epoxymexrenone, useful as a diuretic, and other compounds of Formula Iwherein the variables are as defined by the specification.
Owner:GD SEARLE & CO
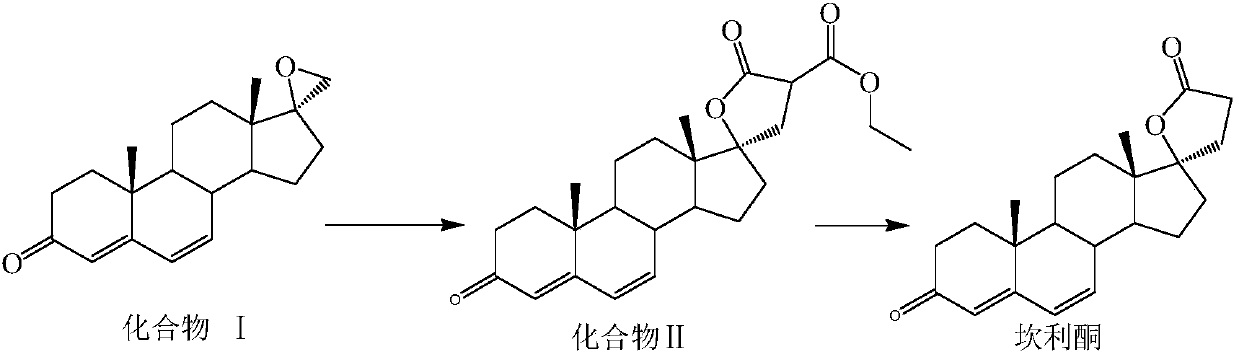
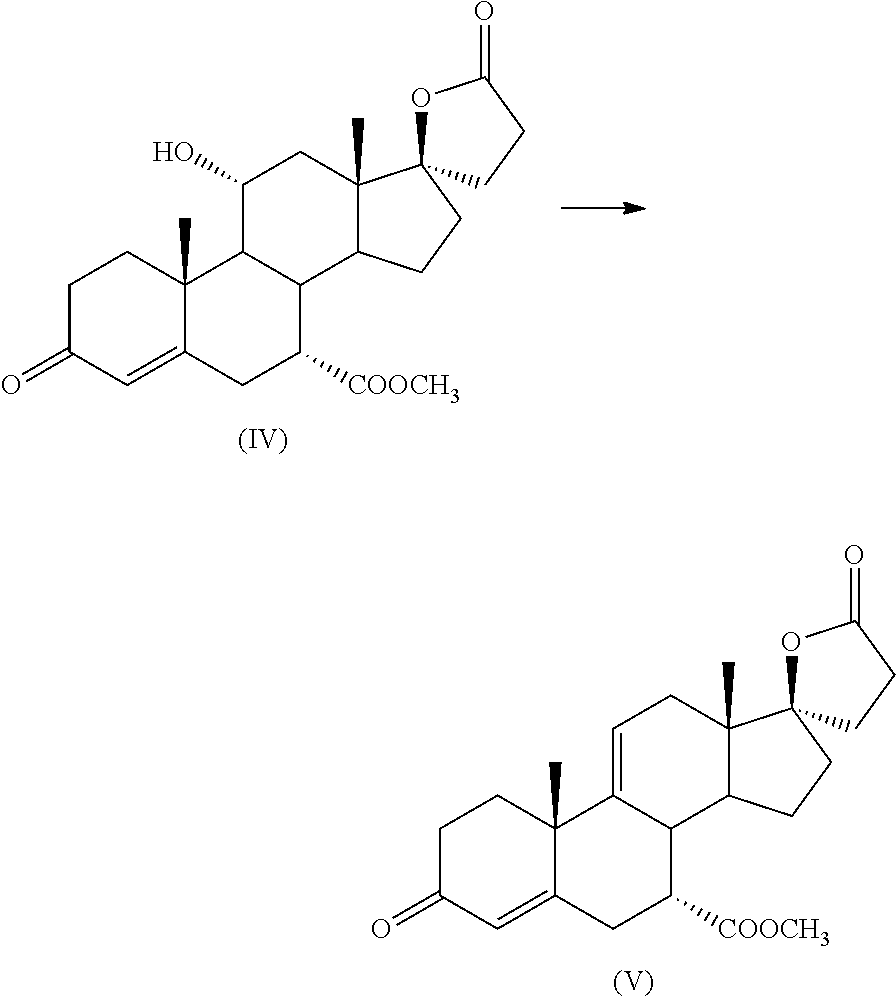
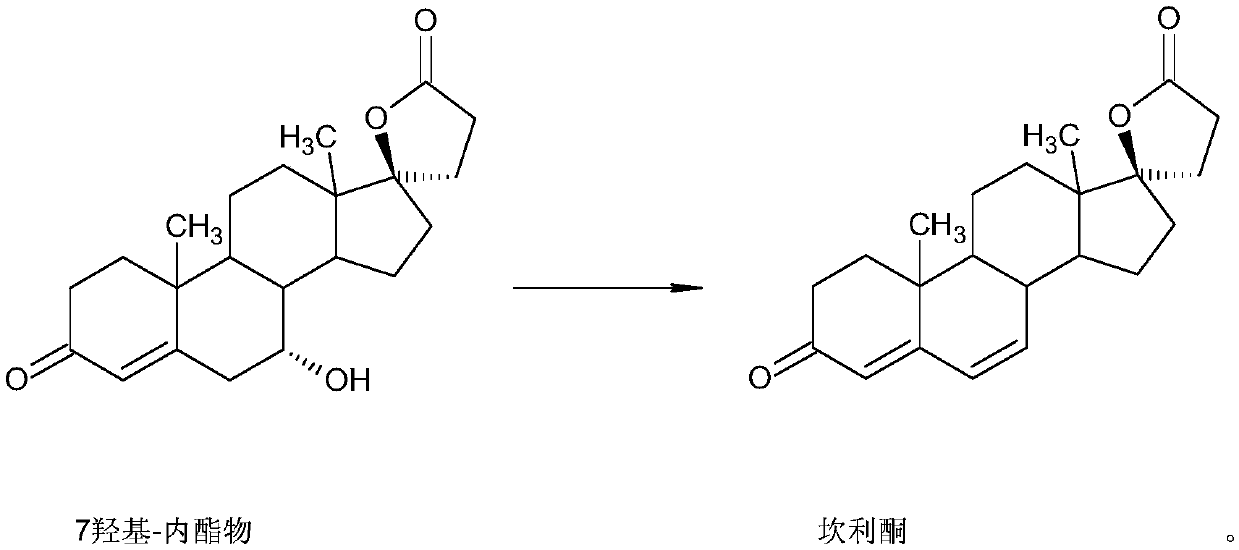
Preparation method for canrenone
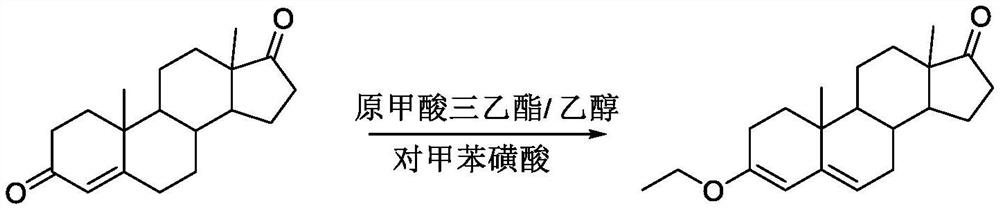
The invention discloses a preparation method for canrenone. According to the preparation method, canrenone is obtained through an etherification reaction and a dehydrogenation reaction, wherein 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone is taken as a raw material; under the presence of a catalyst, the etherification reaction is carried out between 17 beta-hydroxyl-4-alkene-3-ketone-17 alpha-pregnene-21-carboxylic acid-gamma-lactone and triethyl orthoformate to generate 17 beta-hydroxyl-3,5-diene-3-ethoxy-17 alpha-pregnene-21-carboxylic acid-gamma-lactone; the catalyst is pyridine hydrobromide or pyridinium hydrochloride; under the presence of an organic solvent, the dehydrogenation reaction is carried out between an etherification reaction product and an oxidant to generate canrenone; the oxidant is tetrachloro-p-benzoquinone, tetrachloro-o-benzoquinone or 2,3-dichloro-5,6-dicyano-p-benzoquinone. Through the adoption of the preparation method, canrenone of which the purity is 99% or higher can be eventually obtained, and the total weight yield can reach 90% or higher. Therefore, the preparation method is suitable for industrialized production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
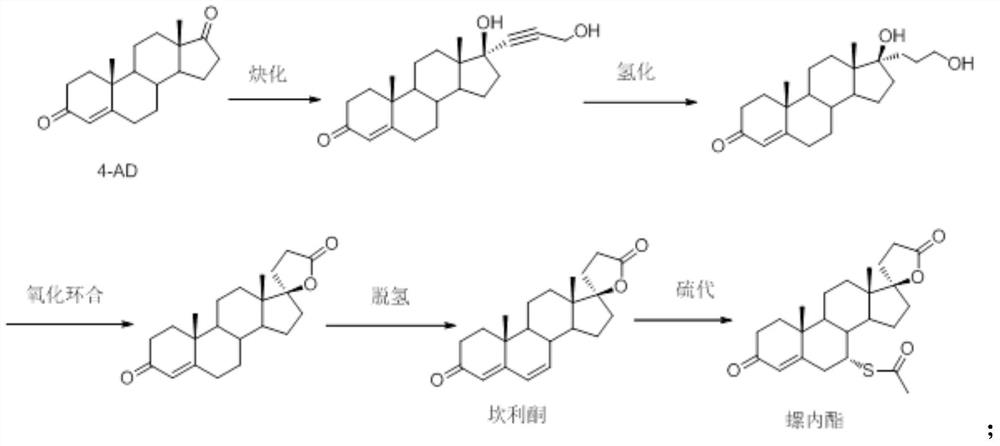
Synthesis method of spironolactone intermediate canrenone
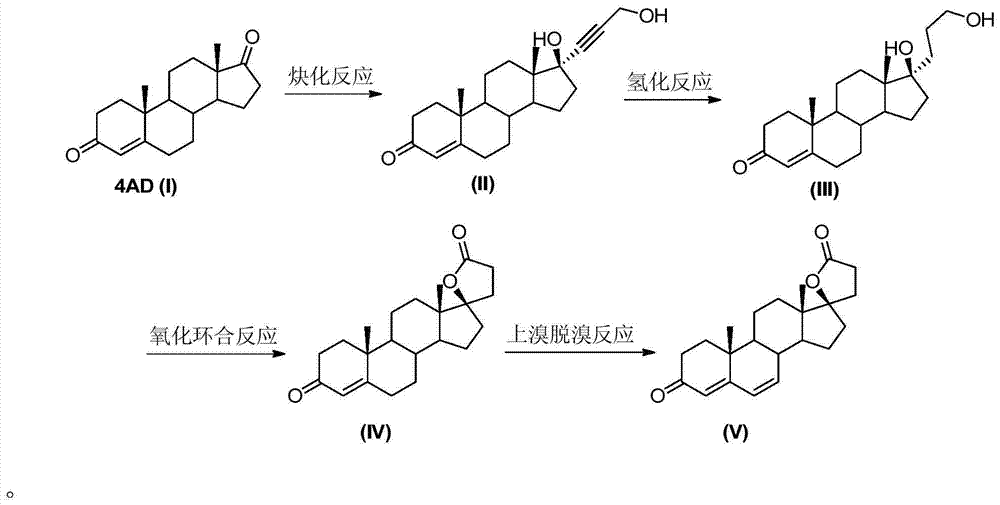
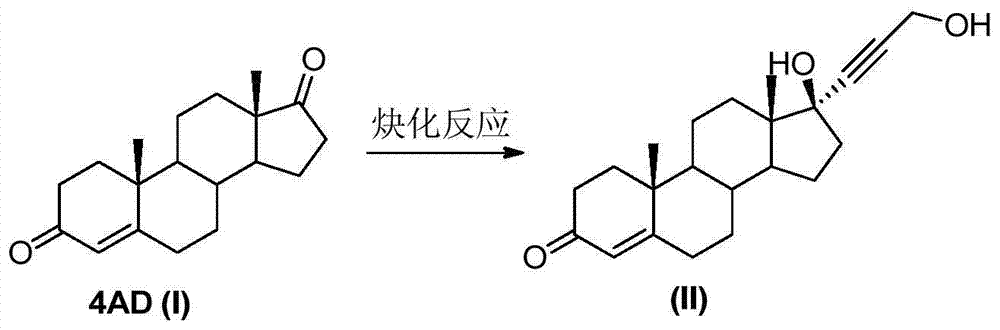
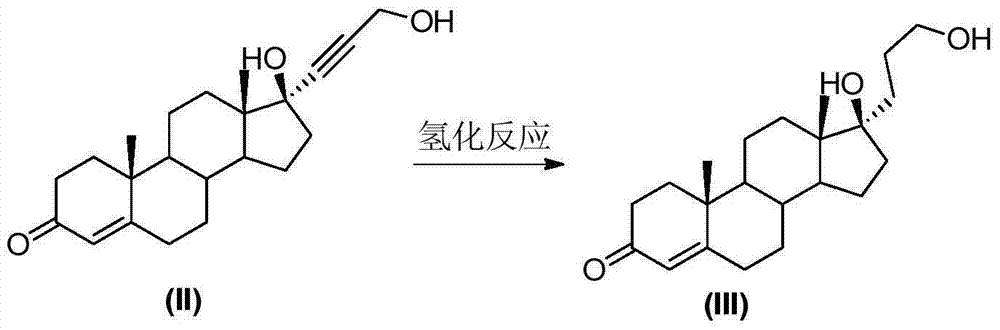
The invention relates to a synthesis method of a chemical medicine, and concretely relates to a synthesis method of a spironolactone intermediate canrenone. The method comprises the following steps: carrying out an ethynylation reaction on a compound I 4-androstenedione (4AD), hydrogenating, carrying out an oxidation cyclization reaction, and carrying out a bromization and debromination reaction to obtain the compound V canrenone, and the above reaction route is shown in the specification. A synthesis method of the structure of an important 21,17-carboxy lactone spiro ring adopted in the invention is different from previous process modes, and is concise and efficient. The method has the characteristics of high yield, good selectivity, low cost, mild reactions, suitableness for industrialization, stability and easy realization.
Owner:ZHEJIANG SHENZHOU PHARMA
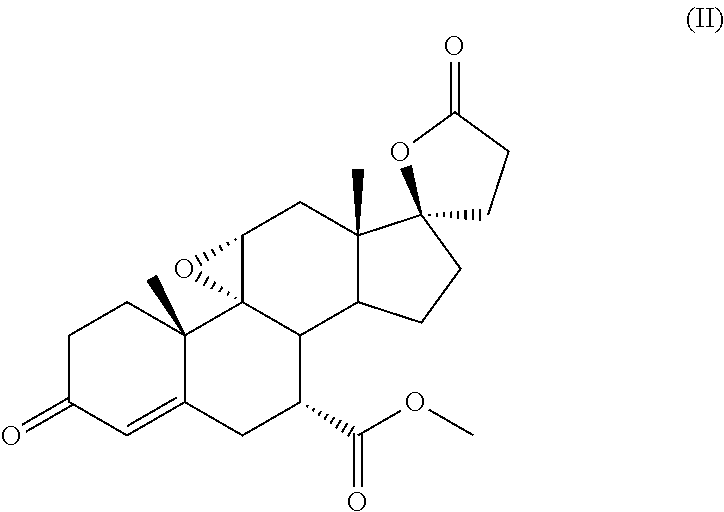
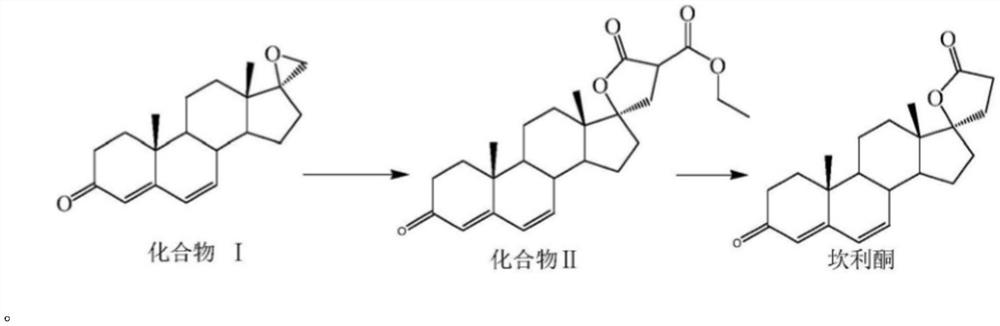
Method for preparing canrenone
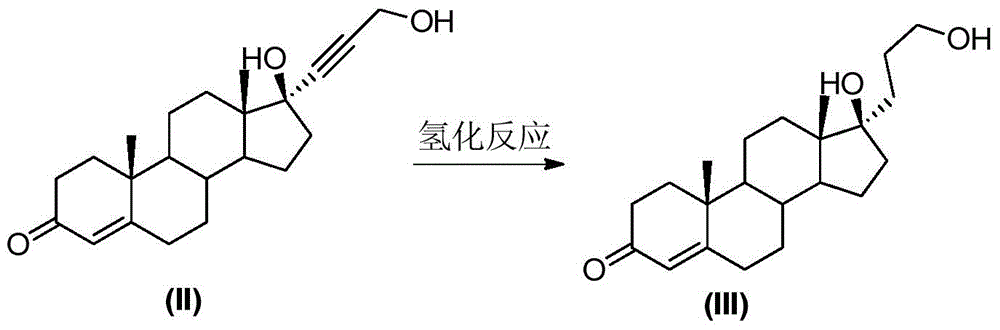
The invention discloses a method for preparing canrenone. The method comprises the following steps of step one, reacting a compound shown in formula (II) with acraldehyde under the action of a catalyst to obtain a compound shown in formula (III); step two, reacting the compound shown in the formula (III) with chloranil to dehydrogenize, thus obtaining the canrenone. The method for preparing the canrenone, provided by the invention, has the advantages of short steps, simplicity and convenience in operation, high synthesis efficiency and suitability for industrial production; a new path is provided for preparing the canrenone.
Owner:JIANGSU MARINE RESOURCES DEV RES INST LIAN YUNGANG
Preparation method of canrenone
The invention provides a preparation method of canrenone. The preparation method comprises the following steps: synthesizing canrenone by using a dehydrogenation product compound I as a substrate, adding a catalyst, carrying out an internal esterification reaction, and optimizing a reaction line. After the reaction is completed, the product is adjusted to be neutral and is directly concentrated, and a high pressure reaction is carried out directly after a dry solvent is recycled by using methylbenzene with ethyl alcohol, so that the aftertreatment reaction steps are reduced, the hydrolysis ofan E-ring ethyl formate group is avoided, and the high-pressure reaction difficulty is greatly lowered. The operability of the reaction is greatly improved, the production cost is reduced, the side reactions are greatly reduced, the reaction of each step is relatively easy to realize, the yield is greatly improved, the production is more economical and safer, and the preparation method is more applicable for industrial production.
Owner:GUANGXI WANDE PHARMA
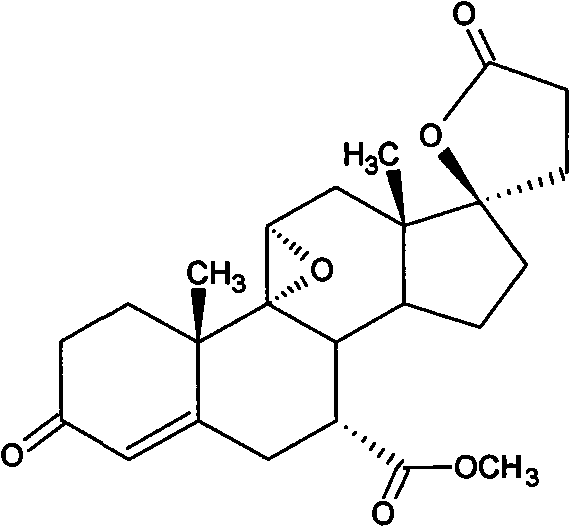
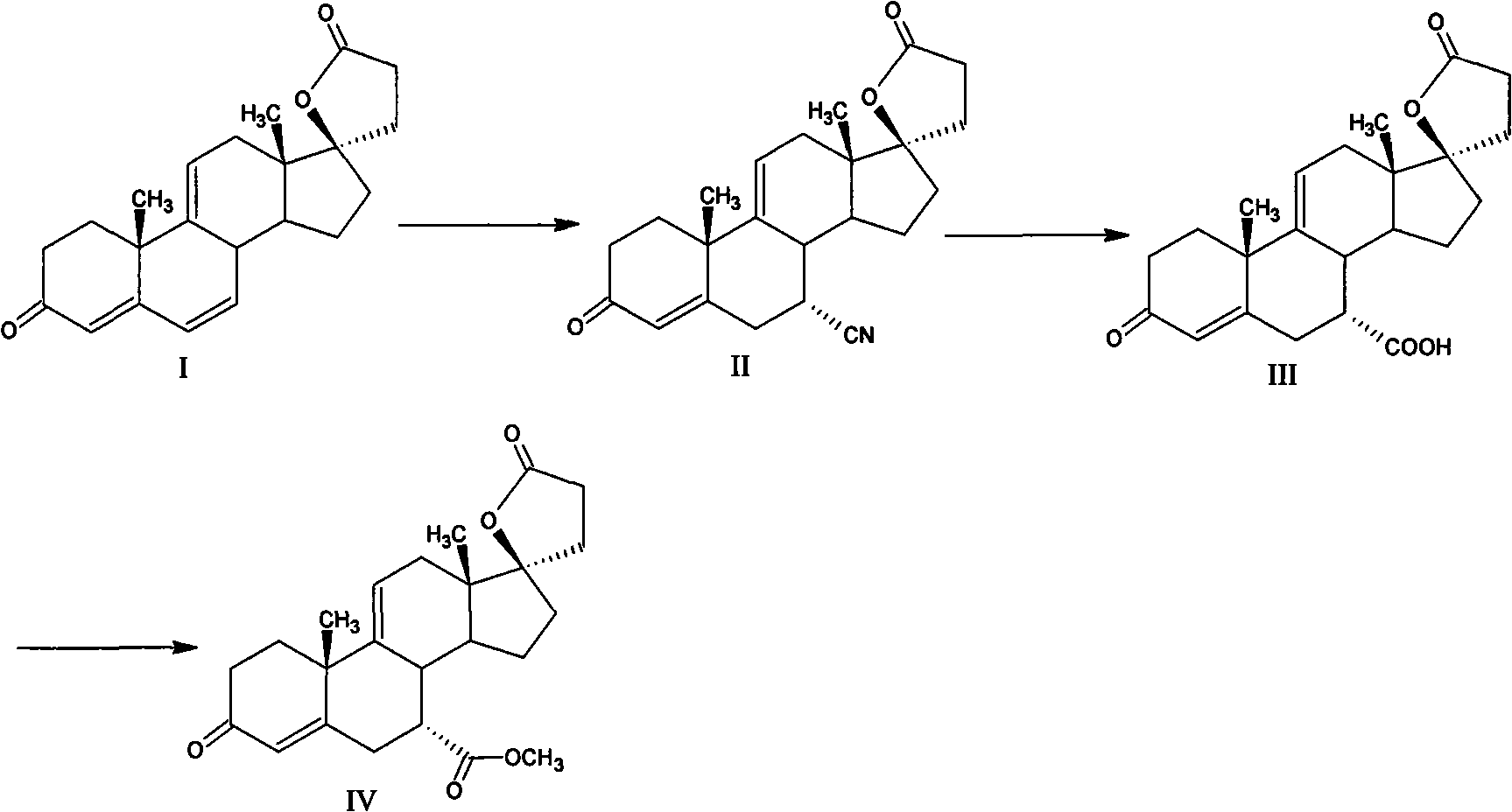
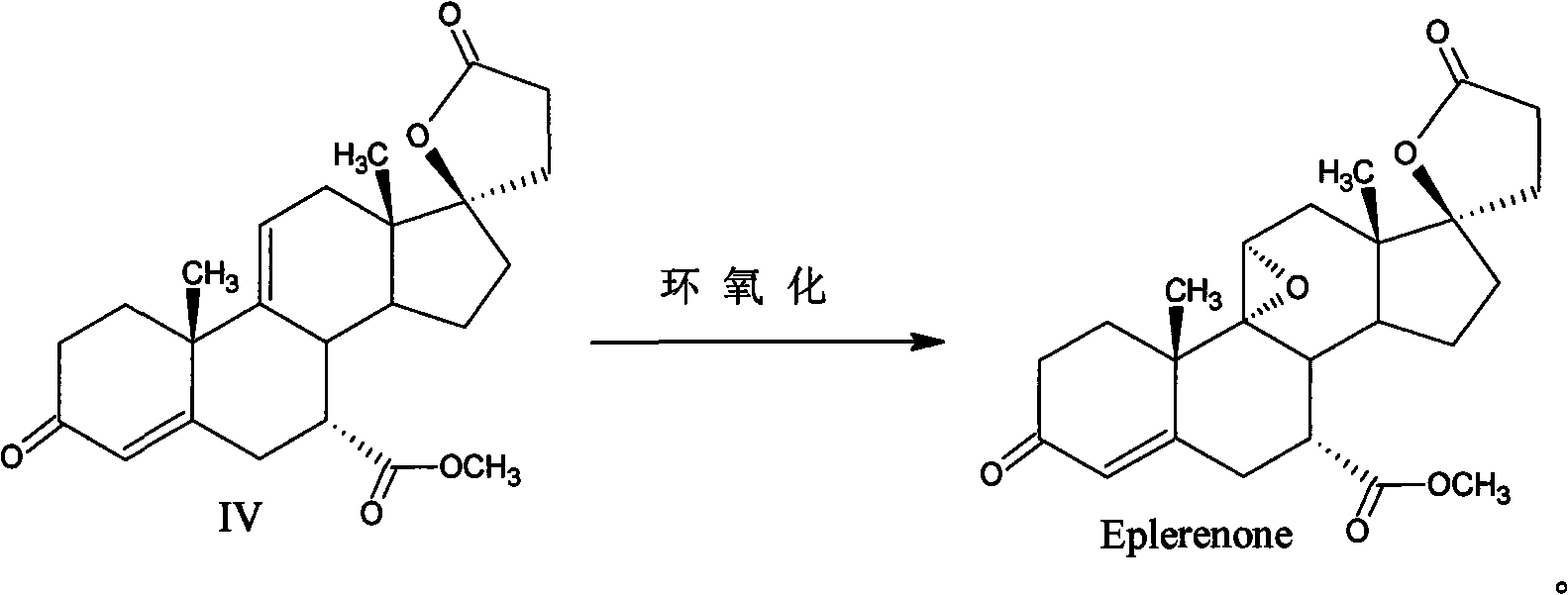
Method for preparing eplerenone
The invention provides a method for preparing eplerenone, which comprises the following steps of: (1) in a solvent, in the presence of a secondary reaction inhibitor, in a buffer system of trichlormethyl eyanide, an oxidizing agent and phosphate, performing double bond selective epoxidation on 17 alpha-hydroxy-3-keto-gamma-lactone-pregna-4,9(11)-diene-7 alpha,21-dicarboxylicacid methyl ester IV to prepare crude eplerenone; and (2) recrystallizing the crude eplerenone to obtain quality eplerenone. The high-purity eplerenone can be prepared by the method, the purity reaches 99.5 percent, the yield reaches 87 percent, and the method is suitable for large-scale industrial production.
Owner:AURISCO PHARMACEUTICAL CO LTD
Novel aldosterone antagonists and uses thereof
InactiveUS20070066580A1Inhibiting aldosterone receptor activityOrganic active ingredientsLactone steroidsIncreased aldosteroneTreatment use
Owner:RECORDATIE IRELAND LTD
Selective glucocorticoid receptor agonists
Owner:UNIV OF MANCHESTER
Imidazolyl progesterone antagonists
Described herein are imidazolyl compounds that exhibit progesterone antagonism without any sign of partial agonistic activity. Such compounds have application in fertility control and in the treatment of hormone dependent breast cancer. The present invention also relates to processes of preparation and the use in therapy of such novel compounds.
Owner:EVESTRA
Locally active "soft" antiandrogens
InactiveUS20140011785A1Reduce occurrenceReduce recurrenceOrganic active ingredientsLactone steroidsChemical structureMedicine
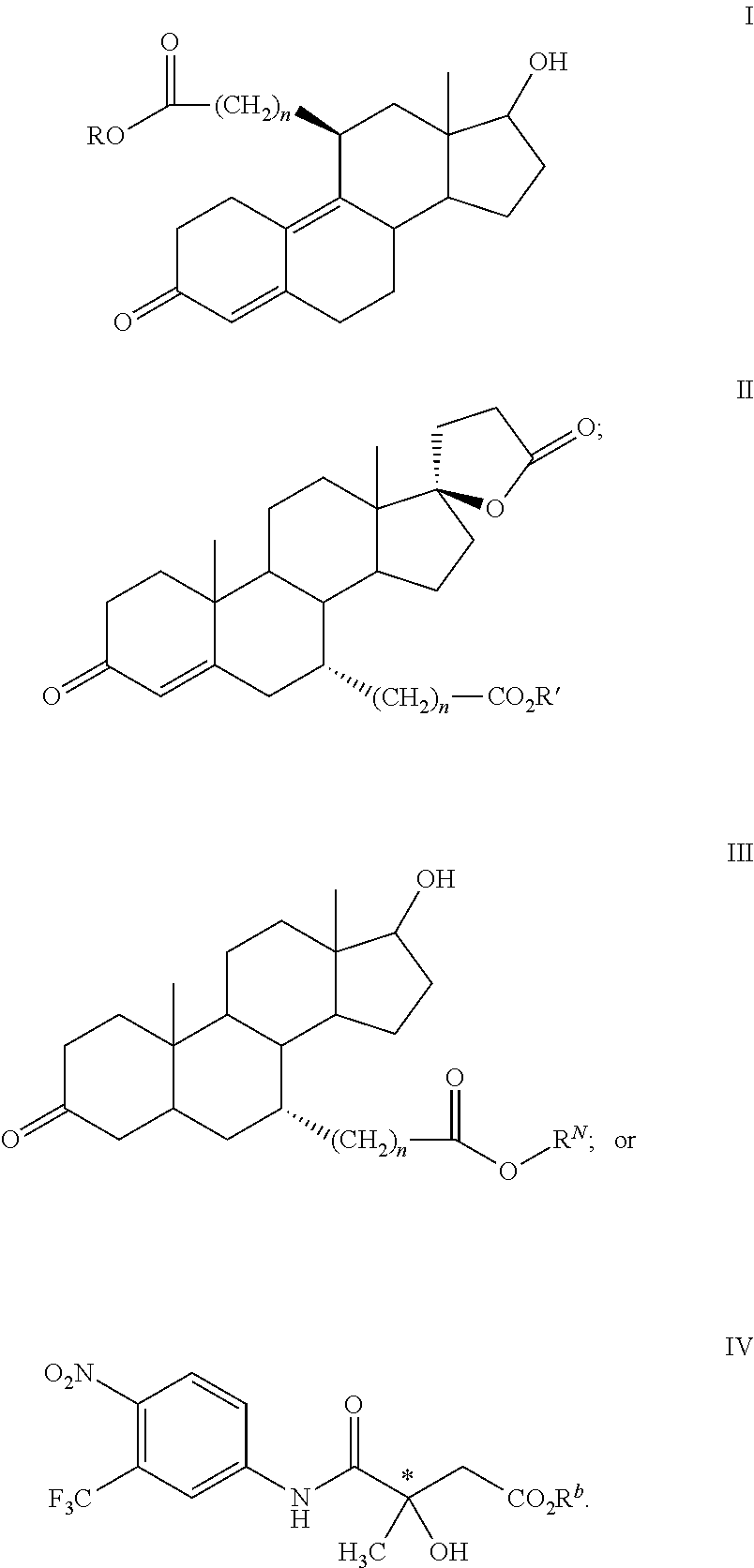
The present invention relates to antiandrogenic compounds which may be administered for the treatment of androgen excess in the skin and by way of consequence, the treatment of acne, baldness or hirsuitism in subject or patient. These compounds have the general chemical structure I, II, III or IV:
Owner:YALE UNIV
Asymmetric synthesis of organic compounds
ActiveUS20140303385A1Silicon organic compoundsOrganic compound preparationMetal catalystChemical compound
The present invention provides processes for the production of chiral compounds in a stereoisomeric excess. The present processes involve reacting a hydrometallated alkene compound with a compound comprising a conjugated-bond system under conditions such that the compounds undergo an asymmetric 1,4- or 1,6-conjugate addition reaction, generating a chiral compound in a stereoisomeric excess. The reaction is performed in the presence of a metal catalyst, which catalyst preferably comprises a non-racemic chiral ligand.
Owner:OXFORD UNIV INNOVATION LTD
Efficient preparation method of delta<9, 11>-canrenone
ActiveCN112062805AReduce process stepsEasy to purifyLactone steroidsDouble bondPharmaceutical Substances
The invention discloses an efficient preparation method of delta<9, 11>-canrenone, and belongs to the technical field of preparation of intermediates of medicines. The method comprises the following steps of: by taking 9 alpha-hydroxyl-4-androstenedione as a raw material, firstly removing 9-site hydroxyl through dehydration reaction to generate delta<9, 11> double bonds, then protecting 3-site carbonyl, then performing epoxidation on 17-site carbonyl, condensing with malonic acid diester to form a lactone ring, and performing oxidative decarboxylation or decarboxylation oxidation reaction to obtain delta<9, 11>-canrenone. According to the method, the raw materials are cheap and easy to obtain, the cost is low, reaction products in all steps are easy to purify, the total mass yield of the final product is higher than 80%, and the method is high in operability, extremely high in commercial competitiveness, suitable for industrial large-scale production and good in economic benefit.
Owner:ZHEJIANG SHENZHOU PHARMA
Asymmetric synthesis of organic compounds
InactiveUS9090545B2Group 4/14 element organic compoundsOrganic compound preparationMetal catalystChemical compound
The present invention provides processes for the production of chiral compounds in a stereoisomeric excess. The present processes involve reacting a hydrometallated alkene compound with a compound comprising a conjugated-bond system under conditions such that the compounds undergo an asymmetric 1,4- or 1,6-conjugate addition reaction, generating a chiral compound in a stereoisomeric excess. The reaction is performed in the presence of a metal catalyst, which catalyst preferably comprises a non-racemic chiral ligand.
Owner:OXFORD UNIV INNOVATION LTD
Dehydrogenation method for preparing canrenone
The invention discloses a dehydrogenation method for preparing canrenone, which comprises the following steps: by taking an intermediate A as a raw material, brominating to obtain a brominated intermediate, then debrominating the brominated intermediate, and adopting calcium bromide and calcium carbonate as debrominating reagents, thereby realizing a dehydrogenation process for clean production of canrenone with high yield and high content. The problem that a large amount of phenol-containing wastewater is generated by dehydrogenation of chloranil is solved.
Owner:SHANDONG SITO BIO TECHNOLOGY CO LTD +1
A kind of synthetic method of spironolactone intermediate canrenone
The invention relates to a synthesis method of a chemical medicine, and concretely relates to a synthesis method of a spironolactone intermediate canrenone. The method comprises the following steps: carrying out an ethynylation reaction on a compound I 4-androstenedione (4AD), hydrogenating, carrying out an oxidation cyclization reaction, and carrying out a bromization and debromination reaction to obtain the compound V canrenone, and the above reaction route is shown in the specification. A synthesis method of the structure of an important 21,17-carboxy lactone spiro ring adopted in the invention is different from previous process modes, and is concise and efficient. The method has the characteristics of high yield, good selectivity, low cost, mild reactions, suitableness for industrialization, stability and easy realization.
Owner:ZHEJIANG SHENZHOU PHARMA
Synthesis process of steroid compound, canrenone and spirolactone
PendingCN111892638ASynthetic conditions are mildHigh molar yieldLactone steroidsMedicineStructural formula
The invention relates to the technical field of medicine synthesis, in particular to a synthesis process of a steroid compound, canrenone and spirolactone. An embodiment of the invention provides thesteroid compound. The steroid compound has a structural formula as shown in the specification. In the structural formula, R is selected from H or an alkyl group. The steroid compound can be used for synthesizing canrenone and spirolactone, synthesis conditions are mild, synthesis efficiency is high, the amount of wastewater is small, the quality of the formed products is high, and production costcan be effectively reduced.
Owner:ZHEJIANG LANGHUA PHARMA
3α,20,20-trihydroxy-5α-pregnant-18-carboxylic acid-γ-lactone and preparation method thereof
ActiveCN112979742BEnhanced inhibitory effectRich varietyLactone steroidsCarboxylic acidMetastatic cell
The invention provides a pregnanoid compound named 3α, 20,20-trihydroxy-5α-pregnant-18-carboxylic acid-γ-lactone, and a preparation method thereof. The present invention has the following technical effects: 1) The inventors of the present invention have unexpectedly discovered a new progesterone compound capable of inhibiting tumor performance, especially for lung cancer cells, ovarian cancer cells, gastric cancer cells and breast cancer high metastatic cells. The good inhibitory effect enriches the types of tumor drugs. 2) The present invention provides a new method for synthesizing pregnanoid compounds. Compared with the existing synthesis methods for pregnanoid compounds, the technical route is relatively shortened, the synthesis method is simple and can be generalized, the reaction conditions are easy to control, and the total yield is improved, which can be improved. for the large-scale preparation of such compounds.
Owner:TAIZHOU POLYTECHNIC COLLEGE
Method for preparing spirolactone intermediate canrenone
ActiveCN113512086AReduce thermal degradationAvoid generatingLactone steroidsPtru catalystPyrrolidinones
The invention provides a method for preparing spirolactone intermediate canrenone. The method comprises the following operation steps: a lactone substance (I) is dissolved in an organic solvent, a catalyst and an auxiliary agent are added, and the mixture is stirred at 50-80 DEG C to prepare the canrenone (II), wherein the organic solvent is at least one of cyclohexane, toluene or methyl tetrahydrofuran; the auxiliary agent is at least one of dimethyl formamide, N,N - dimethyl acetamide and N-methyl pyrrolidone; and the catalyst is poly-4-vinylpyridine. The reaction route is shown in the specification. Compared with the prior art, the method has the advantages that the reaction temperature is low, pressurization is not needed, the quality of canrenone can be improved, the energy consumption can be effectively reduced, and the production cost can be reduced.
Owner:TIANJIN JINJIN PHARMA
A method for synthesizing 7a-methyl formate-9(11)-encanrenone
ActiveCN111018934BShort routeThree wastes less pollutionLactone steroidsFuranBiochemical engineering
The invention discloses a method for synthesizing 7a-methyl formate-9(11)-encanrenone. The method comprises the following steps: reacting 9(11)-encanrenone used as a raw material with 2-methylfuran atfirst, performing ring opening by using dibromohydantoin, rearranging, ozonizing, adding a metal reducing agent to methanol or a mixed solution of methanol and other solvent, and performing reducingesterification to directly obtain the 7a-methyl formate-9(11)-encanrenone. The method has the advantages of operation step simplification, high yield, simplicity in operation, few three wastes, and suitableness for industrial production.
Owner:YANGZHOU LIANAO BIOMEDICAL CO LTD
Crystal of aldactone and preparation method thereof
InactiveCN107501377ALarge granularityThe preparation process is stableOrganic chemistry methodsLactone steroidsFiltrationX-ray
The invention discloses a crystal of aldactone and a crystallization preparation method thereof. X-ray powder has a characteristic peak at diffraction angles 2theta (which is equal to 8.2+ / -0.2, 9.8+ / -0.2, 10.0+ / -0.2, 12.8+ / -0.2, 13.6+ / -0.2, 13.8+ / -0.2, 15.4+ / -0.2, 16.0+ / -0.2, 16.5+ / -0.2, 16.8+ / -0.2, 18.1+ / -0.2, 18.8+ / -0.2, 21.3+ / -0.2, 22.0+ / -0.2, 24.0+ / -0.2 and 25.4+ / -0.2); at the temperature of 30-50 DEG C, aldactone solid is added into good solvent to obtain suspension of which the solution concentration is 0.3-1.0g / ml, and the suspension is put in an ultrasonic system to be processed by ultrasonic wave; poor solvent is added, temperature is lowered to 0-10 DEG C, and crystal grows for 1-5h; vacuum suction filtration, washing and drying are carried out to obtain an aldactone crystal product. A technology is simple, and a recovery rate is 90% or more.
Owner:TIANJIN JINJIN PHARMA
5α-pregna-18,20β-oxygen-3α-ol and its preparation method
ActiveCN112940069BEnhanced inhibitory effectRich varietyLactone steroidsAntineoplastic agentsOncologyMetastatic cell
The present invention provides a pregnant compound named 5α-pregna-18,20β-oxidized-3α-alcohol, and a preparation method thereof. The present invention has the following technical effects: 1) The inventors of the present invention have unexpectedly discovered new pregnantoid compounds capable of inhibiting tumor performance, especially for lung cancer cells, ovarian cancer cells, gastric cancer cells and breast cancer cells with high metastases. Good inhibitory effect enriches the types of tumor drugs. 2) The present invention provides a new synthetic method of pregnant steroids. Compared with the synthetic method of existing pregnant steroids, the technical route is relatively shortened, the synthetic method is simple and scalable, the reaction conditions are easy to control, and the total yield is improved. For the large-scale preparation of such compounds.
Owner:TAIZHOU POLYTECHNIC COLLEGE
Method for preparing canrenone as spironolactone intermediate
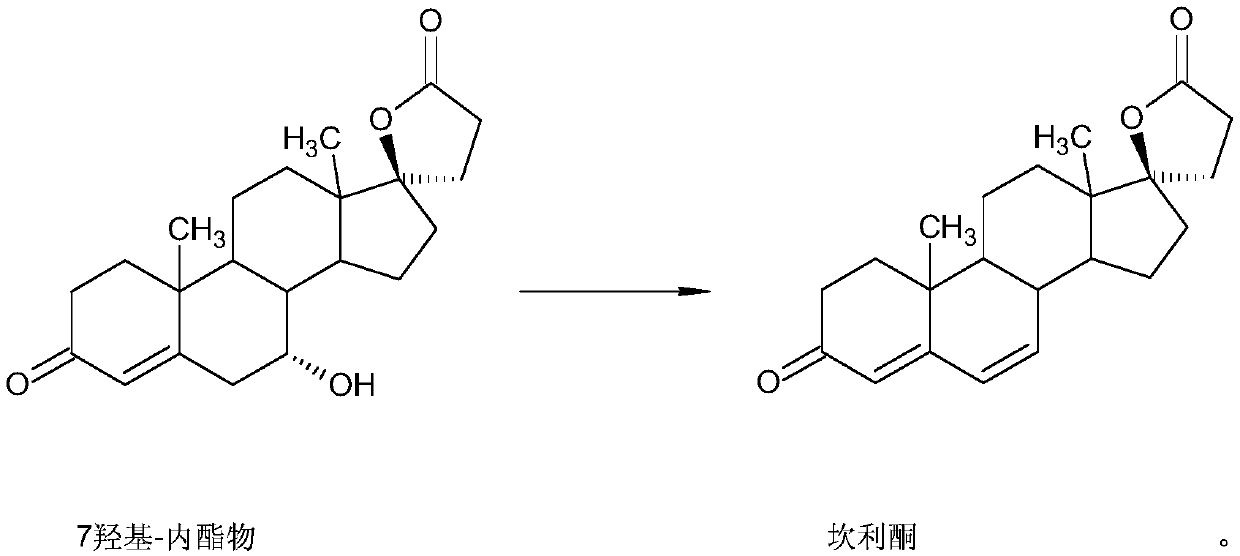
InactiveCN110590895ASimple reaction conditionsNot corrosiveOrganic chemistry methodsLactone steroidsDouble bondFermentation
The invention relates to the technical field of canrenone preparation, and particularly discloses a method for preparing canrenone as a spironolactone intermediate. The method for preparing the canrenone as the spironolactone intermediate specifically comprises the steps that a biological fermentation product 7alpha-hydroxylactone is used as a raw material, a 6,7-site double-bond is formed, and the canrenone as the spironolactone intermediate is obtained. The method for preparing the canrenone as the spironolactone intermediate is simple and efficient and low in the production cost, suitable for large-scale industrial production and convenient for people to use.
Owner:ZHEJIANG SHENZHOU PHARMA
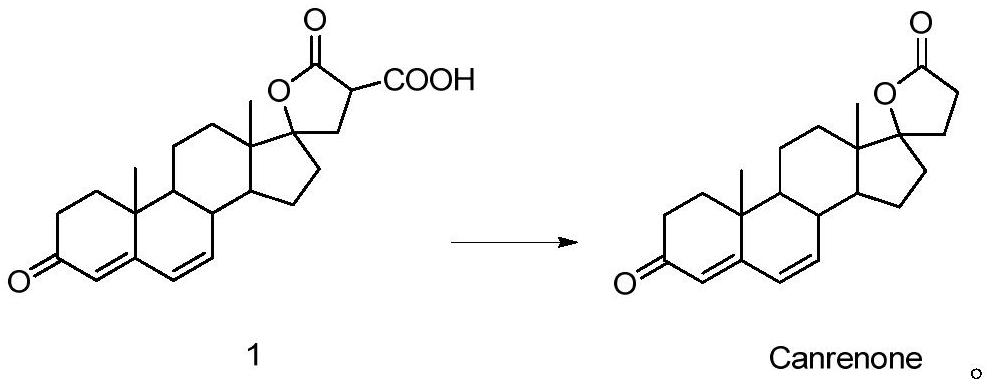
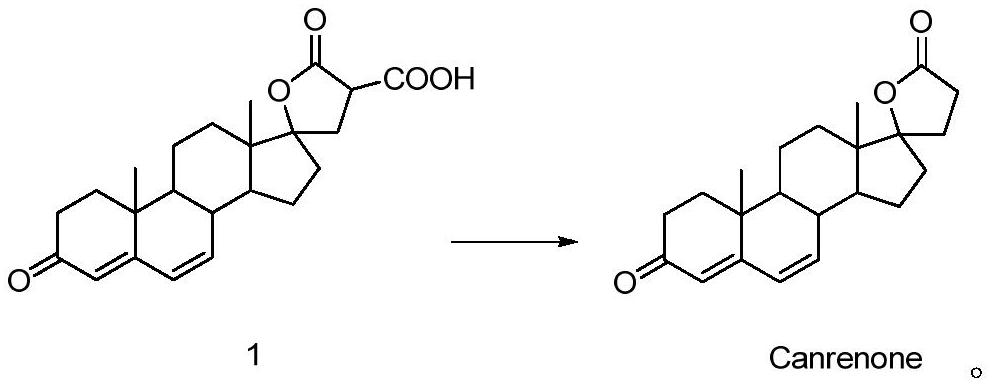
Synthesis method of canrenone
PendingCN113461767ALess side effectsImprove mass transfer efficiencyLactone steroidsChemical synthesisOrganosolv
The invention provides a synthesis method of canrenone, and relates to the technical field of chemical synthesis. The synthesis method of canrenone comprises the following steps: (a) adding a compound in a formula 1 into an organic solvent to obtain a solution containing the compound in the formula 1; and (b) introducing the solution in the step (a) into a micro-channel reactor, and carrying out a decarboxylation reaction to obtain canrenone. The method can completely react within a short time, reduces side reactions caused by long-time high temperature, can continuously react in the microchannel reactor, has the advantages of high mass transfer efficiency, fast reaction, short time and less side reactions, greatly improves the experiment operability, has the yield equivalent to that of the original process, and solves the problems of slow reaction and dangerous and tedious operation, and improves the production applicability of the reaction.
Owner:TIANJIN PHARMA GROUP CORP
Method for preparing eplerenone
ActiveCN101863951BHigh puritySimple methodLactone steroidsCardiovascular disorderPhosphateDouble bond
The invention provides a method for preparing eplerenone, which comprises the following steps of: (1) in a solvent, in the presence of a secondary reaction inhibitor, in a buffer system of trichlormethyl eyanide, an oxidizing agent and phosphate, performing double bond selective epoxidation on 17 alpha-hydroxy-3-keto-gamma-lactone-pregna-4,9(11)-diene-7 alpha,21-dicarboxylicacid methyl ester IV to prepare crude eplerenone; and (2) recrystallizing the crude eplerenone to obtain quality eplerenone. The high-purity eplerenone can be prepared by the method, the purity reaches 99.5 percent, the yield reaches 87 percent, and the method is suitable for large-scale industrial production.
Owner:AURISCO PHARMACEUTICAL CO LTD
Synthesis method of drospirenone
The invention provides a synthesis method of drospirenone, and belongs to the field of organic synthesis. The preparation method comprises the following steps: by taking a compound with a structure as shown in a formula II as an initial raw material, carrying out nucleophilic addition reaction on 17-site carbonyl of the compound and an organic lithium reagent, converting the carbonyl into hydroxyl, introducing propyl methyl ether, then carrying out hydrolysis reaction to obtain a compound with a structure as shown in a formula III, and then under the effect of a noble metal catalyst and persulfate, oxidizing hydroxy on third site into carbonyl, oxidizing hydroxyl on a 17alpha-site propyl group into an aldehyde group, carrying out aldol condensation to form a five-membered carboxylic acid lactone ring to obtain a compound with a structure as shown in a formula IV, and carrying out elimination reaction to obtain the compound drospirenone with a structure as shown in a formula I. The synthesis method provided by the invention has the advantages that most raw materials are cheap, high-pressure catalytic hydrogenation and use of a toxic chromium reagent are omitted, and drospirenone with the yield of 77% and the purity of 99.6% is prepared under mild reaction conditions.
Owner:JIANGXI BAISIKANGRUI PHARMA +1
imidazolyl progesterone antagonist
The present invention relates to imidazolyl compounds which exhibit progesterone antagonism without any signs of partial agonistic activity. These compounds have applications in birth control and the treatment of hormone-dependent breast cancer. The invention also relates to processes for the preparation and therapeutic use of these novel compounds.
Owner:EVESTRA
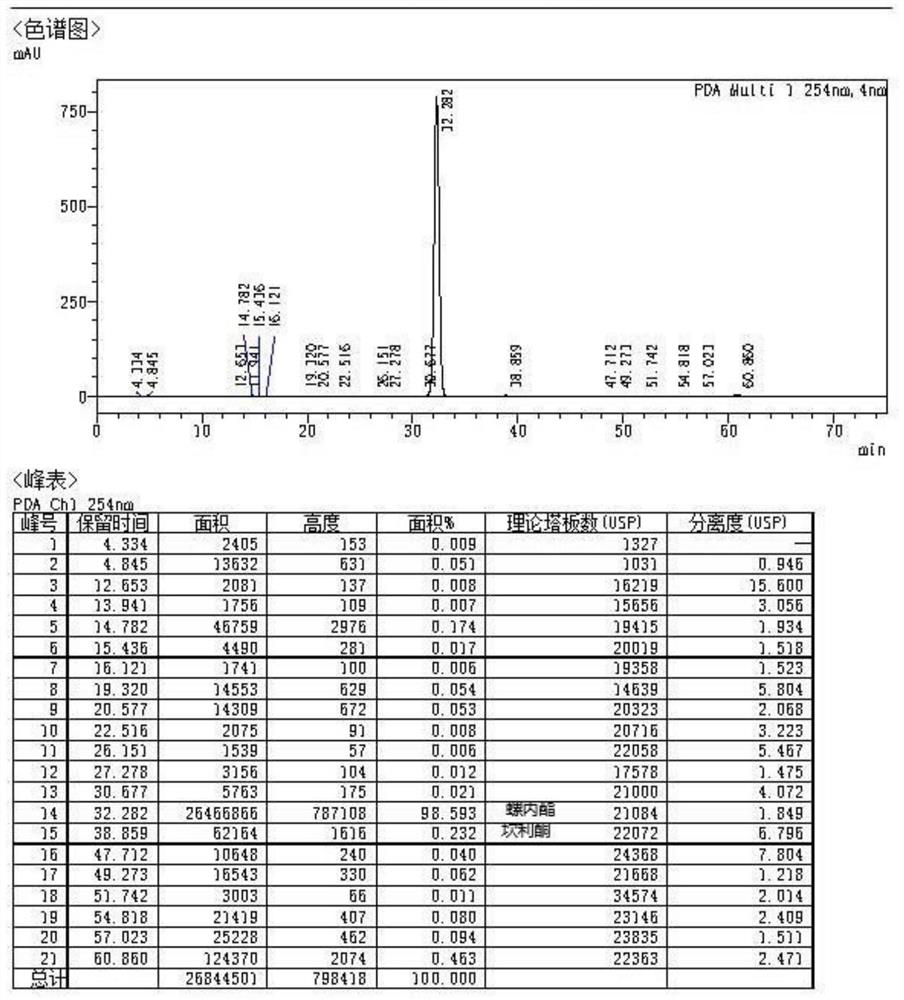
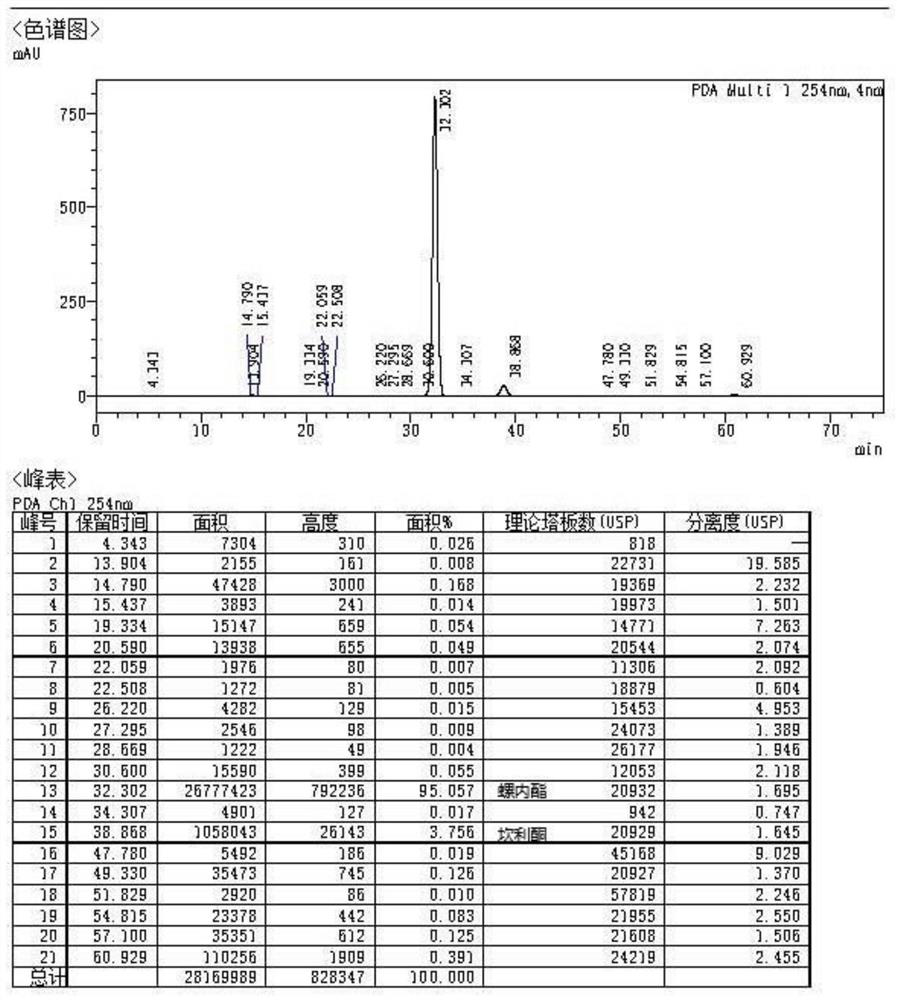
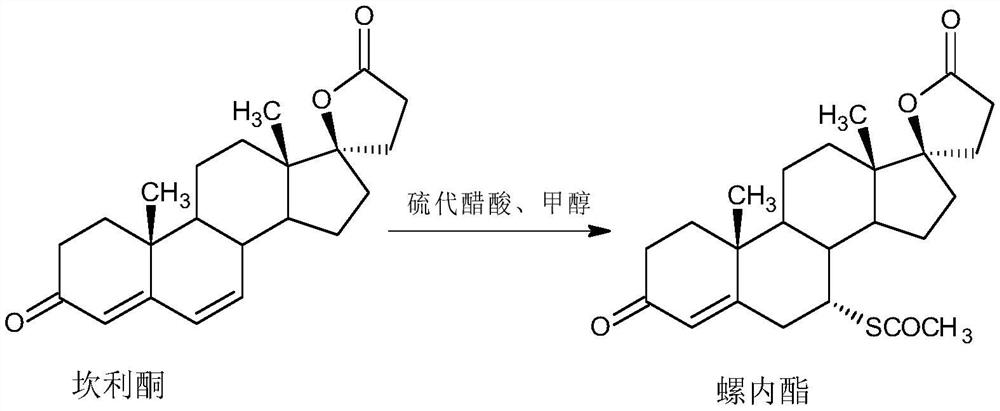
A kind of high-yield spironolactone fine preparation method
The invention discloses a preparation method of a high-yield spironolactone refined product, which belongs to the technical field of medicine preparation and processing. The method uses canrenone as a raw material, reacts with methanol and thioacetic acid to obtain a crude spironolactone, dissolves the crude spironolactone in a solvent, adds a stabilizer, then adds activated carbon, decolorizes, filters out the activated carbon, concentrates and refines to obtain a fine spironolactone. The method of the invention adds a stabilizer in the decolorization process to prevent the spironolactone from degrading into impurities during the decolorization process, improves the yield, has extremely high commercial competitiveness, is suitable for large-scale industrial production, and has good economic benefits.
Owner:ZHEJIANG SHENZHOU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com