Efficient preparation method of delta<9, 11>-canrenone
A high-efficiency technology for canrenone, applied in the field of high-efficiency Δ9,11-canrenone preparation, can solve the problems of increased impurity content, long process route, and reduced yield, and achieve low production cost, reduced process steps, and overall The effect of high quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
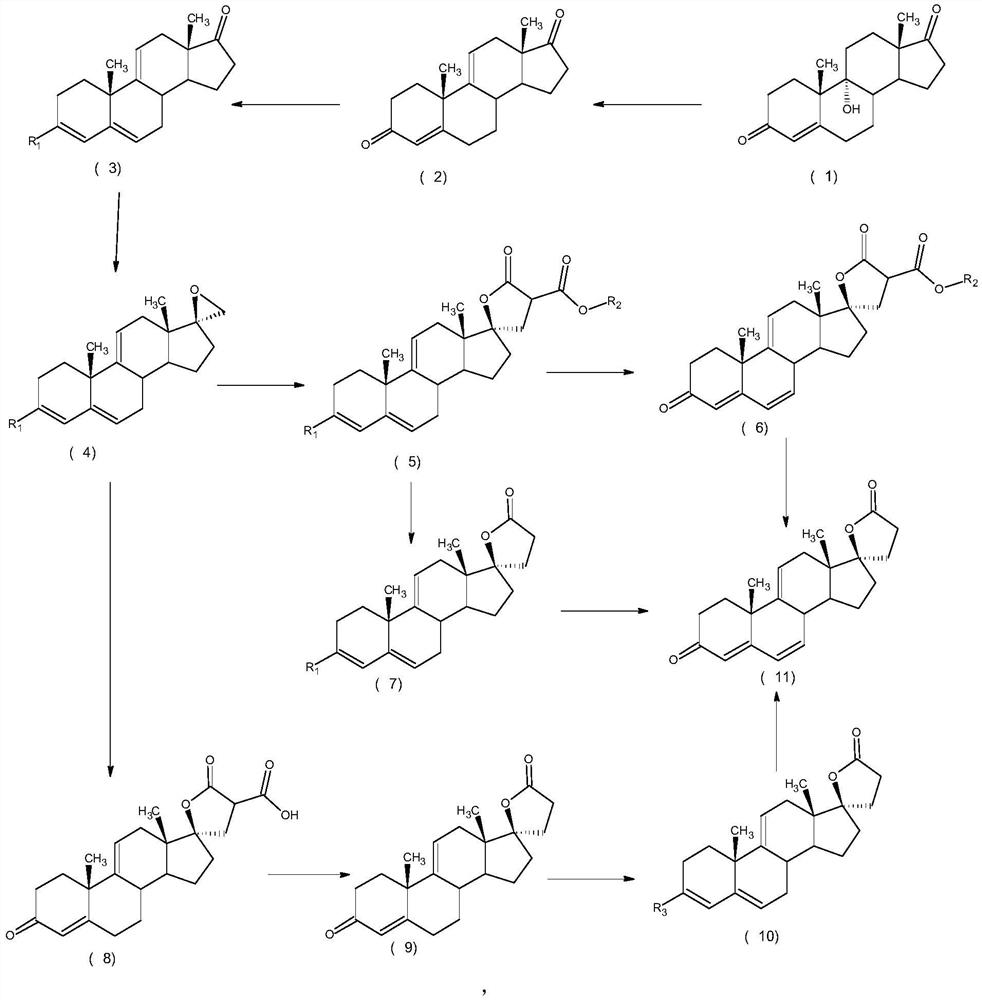
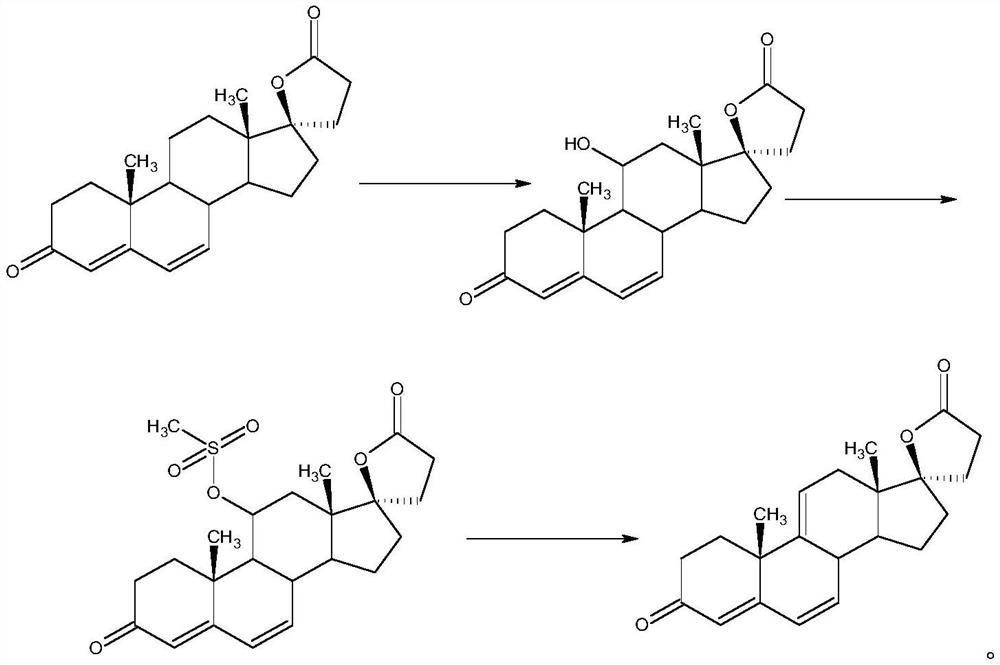
Image
Examples
Embodiment 1
[0034] Example 1Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
[0035] 1) Put 100ml of sulfuric acid solution with a mass concentration of 20% into a three-necked bottle, put in 10g of 9α-hydroxy-4-androstene-3,17dione, and keep warm at 60°C for reaction. Methane extraction, washing with water, concentration, water analysis, filtration, and drying to obtain Δ 9,11 -4-androstene-3,17dione 9.1 g.
[0036] 2) Put Δ 9,11 -4-androstene-3,17dione was put into 4.5ml of methanol, 3.5ml of trimethyl orthoformate and 0.09g of pyridine hydrochloride were added, and the temperature was kept at 20°C for reaction. After the reaction was completed, it was filtered and dried to obtain 3-methanol Oxy-androst-3,5,9(11)-trien-17-one 9.2 g.
[0037] 3) Put 1.2g sodium methoxide and 12.9g trimethylsulfonium iodide into the reaction bottle, 9.2g 3-methoxy-androst-3,5,9(11)-triene-17-one, put 92ml Dimethyl sulfoxide, keep warm at 0°C for reaction, after the reactio...
Embodiment 2
[0041] Example 2Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
[0042] 1) Put 20ml of sulfuric acid solution with a mass concentration of 80% into the three-necked bottle, cool down to 10°C, put in 10g of 9α-hydroxy-4-androstene-3,17dione, keep warm at 10°C for reaction, after the reaction is completed, water analysis , extracted with 200ml dichloromethane, washed with water, concentrated, water analyzed, filtered and dried to obtain Δ 9,11 -4-androstene-3,17dione 9.2 g.
[0043] 2) The 9.2gΔ 9,11 -4-androstene-3,17dione was put into 92ml of tetrahydrofuran, 16.2ml of pyrrolidine and 0.46g of pyridinium hydrobromide were added, and the temperature was kept at 50°C for reaction. After the reaction was completed, it was filtered and dried to obtain 3-pyrrolyl-androstene Ste-3,5,9(11)-trien-17-one 9.48 g.
[0044] 3) Put 5.36g of potassium ethylate and 10.05g of trimethylsulfonium bromide into the reaction bottle, 9.48g of 3-pyrrolyl-androst-3,5,...
Embodiment 3
[0048] Example 3Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
[0049] 1) Put 50ml of sulfuric acid solution with a mass concentration of 50% into a three-neck flask, put in 10g of 9α-hydroxy-4-androstene-3,17dione, and keep warm at 40°C for reaction. After the reaction is completed, analyze with water and extract with 100ml of toluene , washed with water, concentrated, hydrolyzed, filtered, and dried to obtain Δ 9,11 -4-androstene-3,17dione 9.0 g.
[0050] 2) Add 9.0gΔ 9,11 -4-Androstene-3,17-dione was put into 27ml tetrahydrofuran, added 26.4ml triethyl orthoformate, 0.9g p-toluenesulfonic acid, and kept at 40°C for reaction. After the reaction was completed, filtered and dried to obtain 3-ethyl Oxy-androst-3,5,9(11)-trien-17-one 9.0 g.
[0051] 3) Put 10.2g potassium tert-butoxide and 15.3g trimethylsulfonium chloride into the reaction flask, 9g 3-ethoxy-androst-3,5,9(11)-triene-17-one, put 27ml tetrahydrofuran, 27ml dimethyl sulfoxide, ke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com