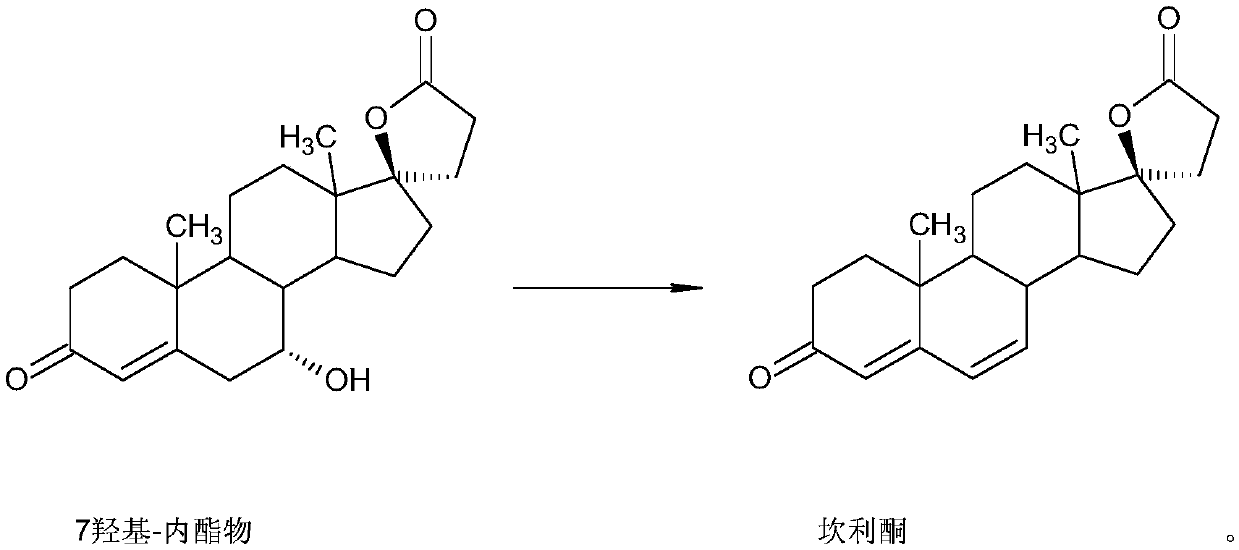
Method for preparing canrenone as spironolactone intermediate
A canrenone and intermediate technology, applied in the field of canrenone preparation, can solve the problems of high corrosiveness, harsh reaction conditions, and high equipment requirements, and achieve the effects of low equipment requirements, simple reaction conditions, and low waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A method for preparing spironolactone intermediate canrenone, the process of the preparation method is:
[0019] Add 5 grams of potassium hydroxide to 70 milliliters of methanol, stir and dissolve under nitrogen protection, then add 10 grams of 7α-hydroxylactone, control the temperature at 5°C for 7 hours, adjust the pH to neutral with acetic acid, concentrate, and add 100 milliliters water, filtered, and refined with methanol to obtain 8.5 g of canrenone.
Embodiment 2
[0021] A method for preparing spironolactone intermediate canrenone, the process of the preparation method is:
[0022] Add 10 grams of 7α-hydroxylactone to 60 ml of dichloromethane, stir and dissolve under nitrogen protection, add 15 ml of triethylamine, control the temperature at about 0°C, add 12 ml of methanesulfonyl chloride dropwise to react for 4 hours, add 50 ml of water The organic layer was washed, dichloromethane was concentrated, and methanol was crystallized to obtain 8.6 g of canrenone.
Embodiment 3
[0024] A method for preparing spironolactone intermediate canrenone, the process of the preparation method is:
[0025] Add 10 g of 7α-hydroxylactone to 50 ml of dichloromethane, stir and dissolve under nitrogen protection, add 18 ml of pyridine, control the temperature at about 5°C, add dropwise 13 ml of trifluoroacetic anhydride to react for 5 hours, add 50 ml of water to wash The dichloromethane layer was concentrated, and crystallized from methanol to obtain 8.4 g of canrenone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com