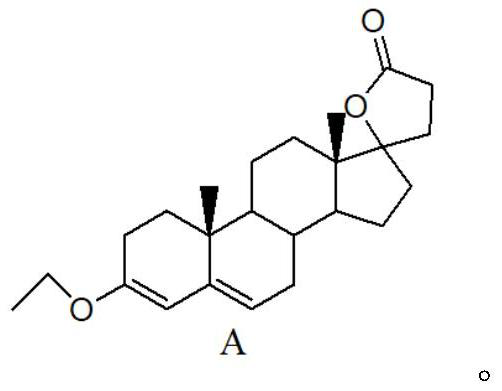
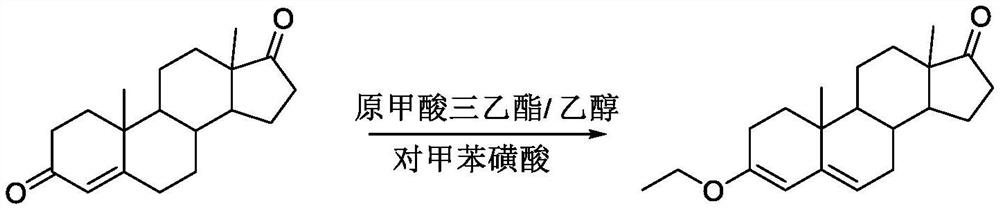
Dehydrogenation method for preparing canrenone
A technology of canrenone and dehydrogenation, applied in chemical instruments and methods, steroids, lactone steroids, etc., can solve the problem of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Bromine: add 100 ml of acetone solvent, 30 g of intermediate A, 0.15 g of anhydrous sodium acetate, N2 protection under ice-salt bath conditions, when the temperature drops below 0 °C, add a total of 15 g in batches N-bromosuccinimide (NBS), and reaction in the dark, after adding 30 minutes, HPLC or TLC monitoring reaction, HPLC monitoring conditions: mobile phase acetonitrile: water=85:15, wavelength=254nm, flow rate=1ml / min, TLC conditions: PE:EA=3:1. After the reaction, add saturated sodium carbonate solution, and filter to obtain the bromide intermediate for use.
[0039] Debromination: Add 15 grams of calcium carbonate and 3 grams of calcium bromide, 100 milliliters of N,N-dimethylformamide, N 2 Replacement and protection, when the temperature rises to 95 ° C, start to add 60 ml of dichloromethane solution of the bromine intermediate dropwise, and continuously distill the dichloromethane solution, and react for 1.5 hours after the dropwise addition is completed. ...
Embodiment 2
[0044] Bromine: add 60 ml of acetone solvent, 30 g of intermediate A, 0.15 g of anhydrous sodium acetate in the reaction flask, and N under ice-salt bath conditions 2 Protection, when the temperature drops below 5°C, add a total of 15 grams of N-bromosuccinimide (NBS) in batches, and react in the dark. After adding for 30 minutes, HPLC or TLC monitors the reaction. HPLC monitoring conditions: Mobile phase acetonitrile: water=85:15, wavelength=254nm, flow rate=1ml / min, TLC condition: PE:EA=3:1. After the reaction, add saturated sodium carbonate solution, and filter to obtain the bromide intermediate for use.
[0045] Debromination: Add 18 grams of calcium carbonate and 3 grams of calcium bromide, 100 milliliters of N,N-dimethylformamide, N 2 Replacement and protection, when the temperature rises to 65 ° C, start to add 60 ml of dichloromethane solution of the bromine intermediate dropwise, and continuously distill the dichloromethane solution, and react for 1.5 hours after the...
Embodiment 3
[0047] Bromine: Add 100 ml of acetone solvent, 30 g of intermediate A, 0.15 g of anhydrous sodium acetate in the reaction flask, and N under ice-salt bath conditions 2 Protection, when the temperature drops below 0°C, add a total of 15 grams of N-bromosuccinimide (NBS) in batches, and react in the dark. After adding for 30 minutes, HPLC or TLC monitors the reaction. HPLC monitoring conditions: Mobile phase acetonitrile: water=85:15, wavelength=254nm, flow rate=1ml / min, TLC condition: PE:EA=3:1. After the reaction, add saturated sodium carbonate solution, and filter to obtain the bromide intermediate for use.
[0048] Debromination: Add 30 grams of calcium carbonate, 100 milliliters of N,N-dimethylformamide, N 2 Replacement and protection, when the temperature rises to 95 ° C, start to add 60 ml of dichloromethane solution of the bromine intermediate dropwise, and continuously distill the dichloromethane solution, and react for 1.5 hours after the dropwise addition is complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com