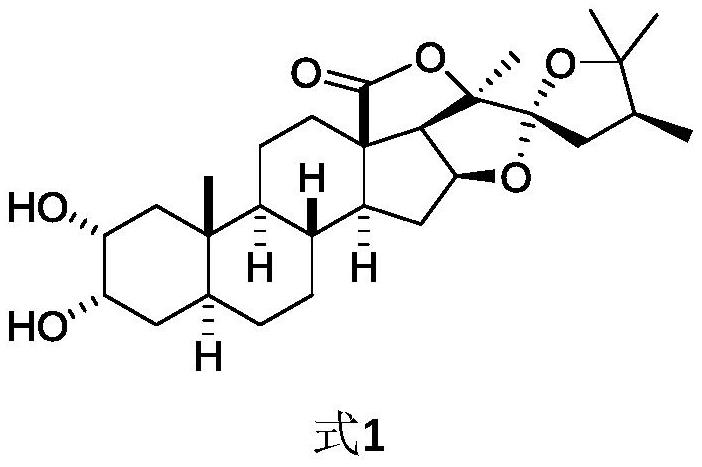
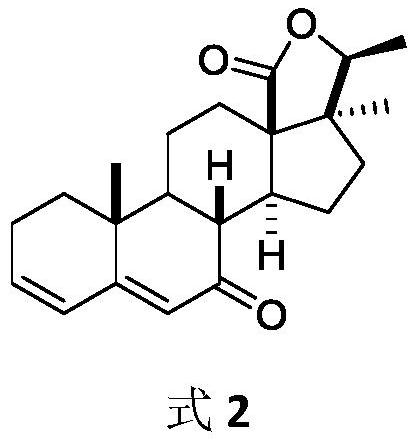
3α,20,20-trihydroxy-5α-pregnant-18-carboxylic acid-γ-lactone and preparation method thereof
A trihydroxy, progesterone technology, applied in the directions of lactone steroids, chemical instruments and methods, steroids, etc., can solve the problems of difficult compound preparation, difficult to use compound preparation in large quantities, long routes, etc., and achieves easy control of reaction conditions, The effect of promoting the synthesis method and shortening the technical route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
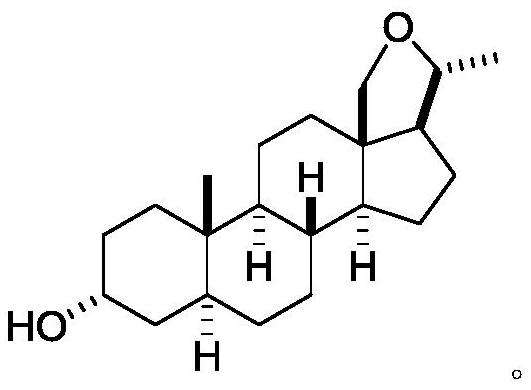
[0108] Example 1-1: Synthesis of 5α-pregnantine--3α,20β-diol (2)
[0109]
[0110] The synthesis method is as follows: 0.62 g of 3α-hydroxy-5α-pregna-20-one (1) (2 mmol) was dissolved in 15 mL of methanol at room temperature, then the solution was cooled to -5 °C, and 0.25 mg of boron was slowly added in four portions. Sodium hydride (6.0 mmol) was added, and the resulting mixture was stirred at -5 °C for 2 h. Subsequently, 1.5 mL of 4M HCl was slowly added dropwise to the reaction system to terminate the reaction, and then extracted with ethyl acetate (2×10 mL). The organic phase of ethyl acetate was washed with 10 mL of saturated brine, dried with 5 g of anhydrous sodium sulfate, and distilled under reduced pressure. The crude product was obtained after removing the solvent, which was purified by column chromatography (silica gel, eluent: 35% ethyl acetate-petroleum ether (60-90°C)) to give the product 0.45g 5α-pregna-3α,20β-diol (2), the yield was 69%. 1 H NMR (CDCl 3...
Embodiment 2-1
[0111] Example 2-1: Synthesis of 18-iodo-5α-pregnant-3α,20β-diol (3)
[0112]
[0113] At room temperature, 0.65g of 5α-pregnant-3α, 20β-diol (2) (2mmol) was dissolved in 180mL of anhydrous petroleum ether, and then 1.02g of iodophenyl acetate (3mmol) was slowly added to the solution, resulting in The suspension was deaired with nitrogen for 15 minutes. Subsequently, 626 mg of iodine (2.5 mmol) was slowly added dropwise to the reaction system, and the reaction system was irradiated with a 300W tungsten lamp for 1 hour at a reaction temperature of 25°C, and then irradiated with a 300W tungsten lamp at 70°C for 30 minutes. After the reaction, it was cooled to room temperature, transferred to a separatory funnel, washed with 6 ml of 10% sodium thiosulfate, washed with 10 ml of saturated brine, dried with 5 g of anhydrous sodium sulfate, and distilled under reduced pressure to remove the solvent to obtain a crude product , purified using column chromatography (silica gel, elue...
Embodiment 3-1
[0114] Example 3-1: Synthesis of 5α-pregnant-18,20β-oxo-3α-ol (4)
[0115]
[0116] 0.89 g of 18-iodo-5α-pregna-3α,20β-diol (3) (2 mmol) was dissolved in 10 mL of anhydrous tetrahydrofuran, and 0.22 g of potassium tert-butoxide (2 mmol) was slowly added. It was then heated (oil bath temperature 76°C) and stirred for 12 hours. After the reaction, the solvent was evaporated, 10 mL of distilled water was added to the residue, and then ethyl acetate (2×10 mL) was used for extraction. The ethyl acetate organic phase was washed with 10 mL of saturated brine, dried with 6 g of anhydrous sodium sulfate, and the solvent was removed by distillation under reduced pressure. The crude product was then obtained and purified using column chromatography (silica gel, eluent: 35% ethyl acetate-petroleum ether (60-90°C)) to give the product 0.45g 5α-pregnant-18,20β-oxo-3α- Alcohol (4) in 71% yield. 1 H NMR (CDCl 3 ,400MHz)δ: 4.04(d,1H,J=2.5Hz,C 3β -H),3.74(d,1H,J=3Hz,C 20 -H),3.71(d,2H J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com