Quinolone thiazole compound and preparation method and application thereof
A quinolone thiazole and quinolone technology, applied in the field of chemistry, can solve problems such as toxic and side reactions, achieve the effects of high synthesis yield, easy availability of raw materials, and solving drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
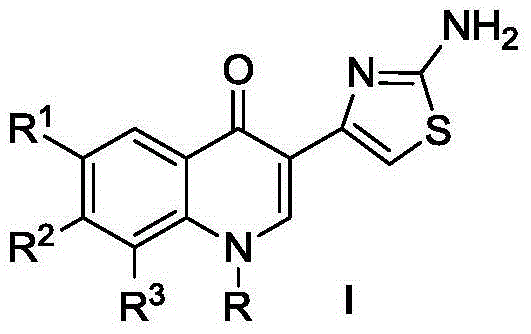
[0026] The preparation of the compound represented by the general formula II: take ethyl acetoacetate and triethyl orthoformate as raw materials to undergo nucleophilic substitution reaction to obtain an intermediate, and then use diphenyl ether as a solvent to obtain 3- The quinolone ring with the acetyl group, followed by triethylamine as the catalyst and dimethyl sulfoxide as the solvent, undergoes nucleophilic substitution on the benzene ring at 120°C to obtain the intermediate of the secondary amine substituent, and then uses the basic catalyst DMF Reaction with ethyl bromide or cyclopropyl bromide as a solvent at 100°C to obtain the corresponding intermediate, and then brominated under the condition of acetic acid as a solvent to obtain the compound represented by the general formula II.
[0027]
Embodiment 1
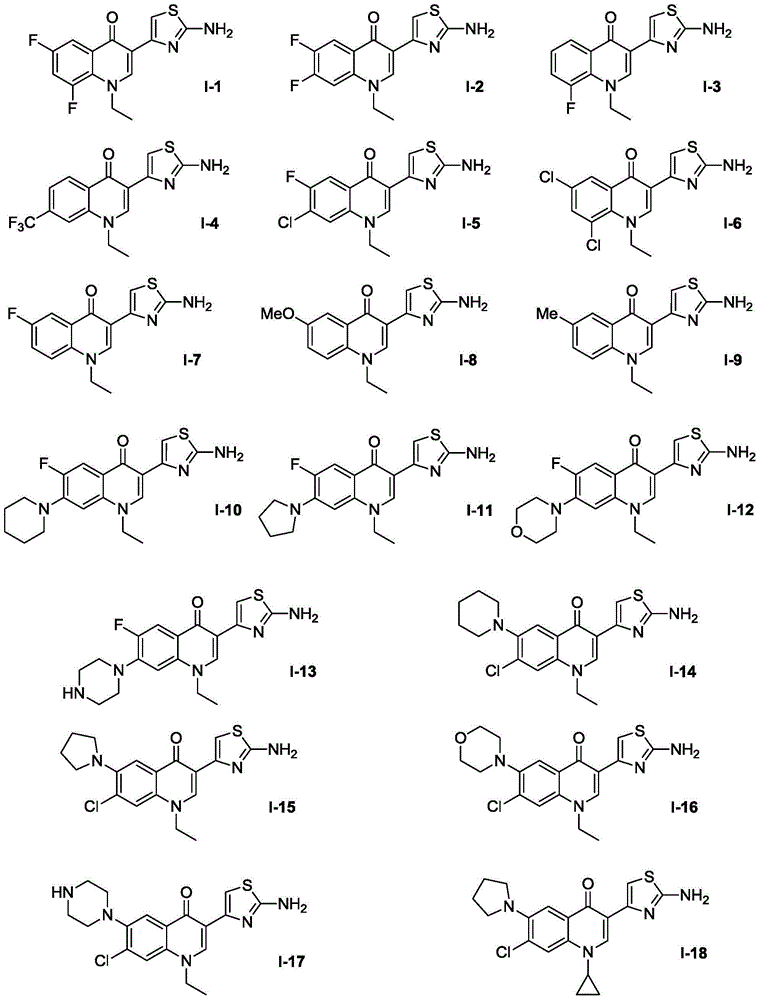
[0028] Embodiment 1, the preparation of compound I-1
[0029]
[0030] In a 100mL round-bottomed flask, compound II-1 (0.33g, 1mmol), thiourea (0.076g, 1mmol) and ethanol (30mL) were stirred and reacted at 60°C for 3h under temperature control, cooled to room temperature, and traced by TLC to After the reaction was completed, 0.246 g was obtained by concentration, extraction, column chromatography separation, recrystallization and drying, with a yield of 80.0%. Wherein compound II-1: R is ethyl, R1 is fluorine, R2 is hydrogen; R3 is fluorine.
[0031] Compound I-1: yellow powder; melting point: 240-242°C; IR (KBr, cm -1 )ν: 3423(N-H), 3170(Ar-H), 3051(=C-H), 2989, 2865(CH 2 ,CH 3 ), 1656 (C=O), 1609, 1580, 1501, (aromatic frame); 1 H NMR (600MHz, CDCl 3 , ppm) δ8.59 (s, quinolone-2-H, 1H), 8.09 (d, J=7.5Hz, quinolone-5-H, 1H), 7.94 (s, thiazole-5-H, 1H), 7.18 (ddd, J=14.1,7.5,2.7Hz,quinolone-7-H,1H),4.98(s,thiazole-2-NH 2 ,2H),4.43(tt,J=6.9,3.5Hz,CH 2 CH 3 ,2H),1.5...
Embodiment 2
[0032] Embodiment 2, the preparation of compound 1-2
[0033]
[0034] In a 100mL round-bottomed flask, compound II-2 (0.33g, 1mmol), thiourea (0.076g, 1mmol) and ethanol (30mL) were stirred and reacted at 60°C for 3h under temperature control, cooled to room temperature, and traced by TLC to After the reaction was completed, 0.261 g was obtained by concentration, extraction, column chromatography separation, recrystallization and drying, with a yield of 85.1%. Wherein compound II-2: R is ethyl, R1 is fluorine, R2 is fluorine; R3 is hydrogen.
[0035] Compound I-2: yellow powder; melting point: 238-240°C; IR (KBr, cm -1 )ν: 3420(N-H), 3170(Ar-H), 3050(=C-H), 2995, 2865(CH 2 ,CH 3 ), 1655 (C=O), 1609, 1580, 1501, (aromatic frame); 1 H NMR (600MHz, CDCl 3 ,ppm): δ8.64(s,quinolone-2-H,1H),8.35(dd,J=10.7,9.0Hz,quinolone-5-H,1H),7.95(s,thiazole-5-H,1H ), 7.31–7.27 (m, quinolone-8-H, 1H), 4.85 (s, thiazole-2-NH 2 ,2H),4.23(q,J=7.3Hz,CH 2 CH 3 ,2H),1.55(t,J=7.2Hz,CH 2 CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com