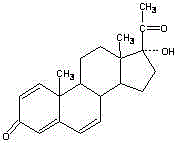
A kind of synthetic method of cyproterone acetate dehydrogenate
A technology of cyproterone acetate and a synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of serious environmental pollution, high consumption of auxiliary materials, low synthesis yield and the like, and achieves reduction of environmental pollution, reduction of production costs, The effect of shortening the reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put in 25g of 17α-OH progesterone, 40g of absolute ethanol, and 15.75g of triethyl orthoformate, stir for 30 minutes (incompletely dissolved, white suspension), quickly add 0.88g of pyridinium p-toluenesulfonate, heat up to 38°C, keep warm After 6.5 hours of reaction, the TLC detection was almost complete (developing agent: benzene: acetone = 6: 1), lower the temperature below 25°C, add 0.88g of triethylamine to neutralize to acidity (PH = 7-8), and stir for 10 minutes.
[0021] Drop into 216.5g toluene in the above-mentioned reactant, and pass into N 2 Protect, stir for 10 minutes, then add 30g of dichlorodicyanoquinone, stir to raise the temperature to 90°C, and keep stirring at a constant temperature of 85°C for 3.5h, sample TLC to analyze the complete reaction of raw materials (developer: acetone: chloroform = 1:20).
[0022] Cool down to 60°C, filter while hot, and wash the filter cake with a small amount of hot toluene; combine the filtrate and lotion, wash with 5...
Embodiment 2
[0025] Add 25g of 17-OH progesterone, 41.25g of absolute ethanol, and 17g of triethyl orthoformate, stir for 30 minutes (incompletely dissolved, white suspension), quickly add 1.0g of pyridinium p-toluenesulfonate, heat up to 40°C, and keep warm After 6.5 hours of reaction, the TLC detection is basically complete (developing agent: benzene: acetone = 6: 1), lower the temperature below 25 °C, add 1.0 g of triethylamine to neutralize to acidity (PH = 7-8), and stir for 10 min.
[0026] Drop into 217.75g toluene in the above-mentioned reactant, and pass into N 2 Protect and stir for 10 minutes; then add 31.3g of dichlorodicyanoquinone, stir to raise the temperature to 90°C, and stir at a constant temperature of 88°C for 3.5h, sample TLC to analyze the complete reaction of raw materials (developer: acetone: chloroform = 1:20).
[0027] Cool down to 60°C, filter while hot, and wash the filter cake with a small amount of hot toluene; combine the filtrate and lotion, wash with 50ml o...
Embodiment 3
[0030] Add 25g of 17α-OH progesterone, 30g of absolute ethanol, and 10g of triethyl orthoformate, stir for 30 minutes (incompletely dissolved, white suspension), quickly add 0.25g of pyridinium p-toluenesulfonate, heat up to 42°C, and keep warm for reaction After 6.5 hours, the TLC detection was almost complete (developer: benzene:acetone = 6:1), lower the temperature below 25°C, add 0.3g triethylamine to neutralize to acidity (PH=7-8), and stir for 10 minutes.
[0031] Drop into 150g toluene in the above-mentioned reactant, and pass into N 2 Protect, stir for 10 minutes, then add 20g of dichlorodicyanoquinone, stir to raise the temperature to 90°C, and keep stirring at 90°C for 3.5h, sample TLC to analyze the complete reaction of raw materials (developer: acetone: chloroform = 1:20).
[0032] Cool down to 60°C, filter while hot, and wash the filter cake with a small amount of hot toluene; combine the filtrate and lotion, wash with 50ml of water, and concentrate the toluene fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com