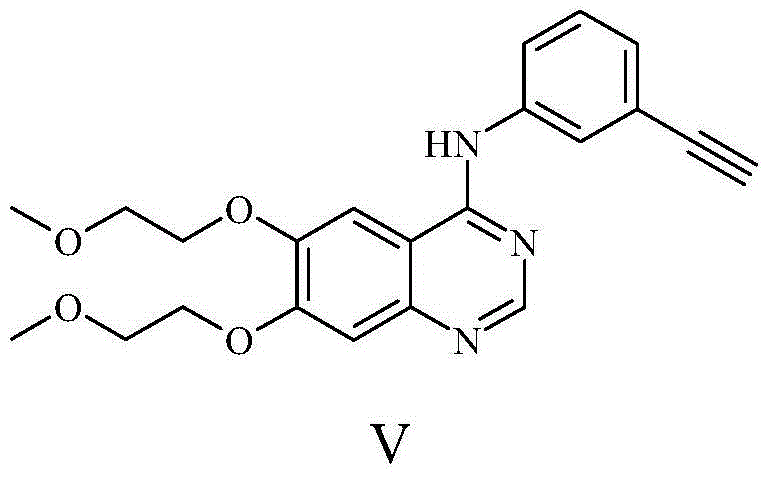
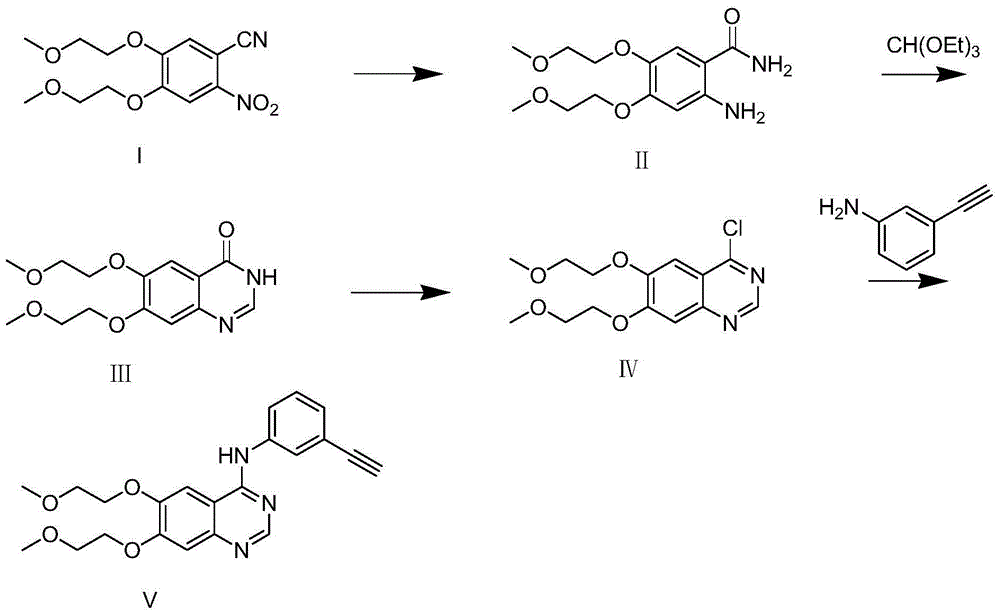
Preparation method of erlotinib
A technology of erlotinib and methoxyethoxy, which is applied in the field of preparation of erlotinib, can solve the problems of long reaction steps, low nitration yield and purity, increased reaction cost, etc., and achieves mild reaction conditions, The effect of shortening the reaction steps and reducing the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
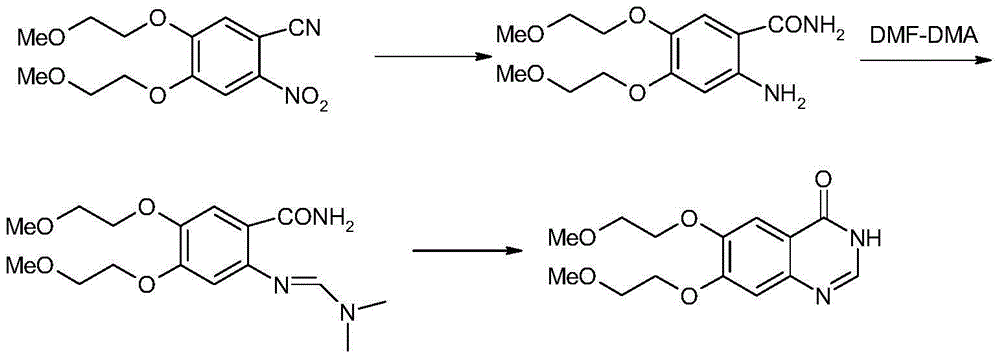
[0032] (1) Synthesis of compound II (2-amino-4,5-bis(2-methoxyethoxy)benzamide).
[0033] At 0°C, suspend 214g of compound I (4,5-bis(2-methoxyethoxy)-2-nitrobenzonitrile) and 50g of Raney Ni in about 2L of water, stir mechanically, add dropwise 141mL of hydrated The aqueous solution of hydrazine was 500mL, after the dropwise addition was completed, it was raised to room temperature (about 20-30°C), and reacted for 3h, monitored by TLC until the reaction was completed. Add sodium hydroxide solution to adjust the pH value to between 11-12, add about 2L of dichloromethane to extract twice, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, and rotary evaporate to obtain 170 g of crude compound II. The yield is 83%.
[0034] The characterization data of this compound II are: 1 H-NMR (400MHz, DMSO-d6): 7.15 (1H, s), 6.40 (2H, s), 6.27 (1H, s), 5.73 (1H, s), 4.01-4.03 (2H, J=4.8Hz, t),3.95-3.98(2H,J=4.8Hz,t),3.64-3.66(2H,J=4.8Hz,t),3.58-3.60(2H,J=4.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com