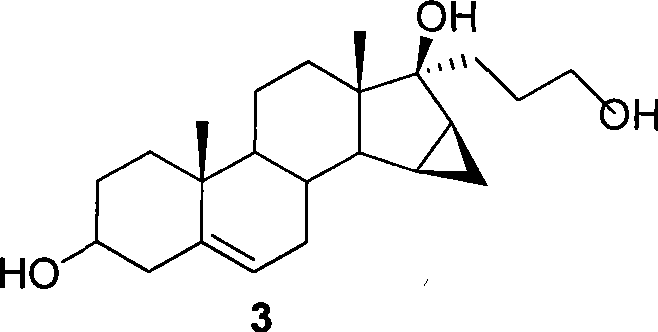
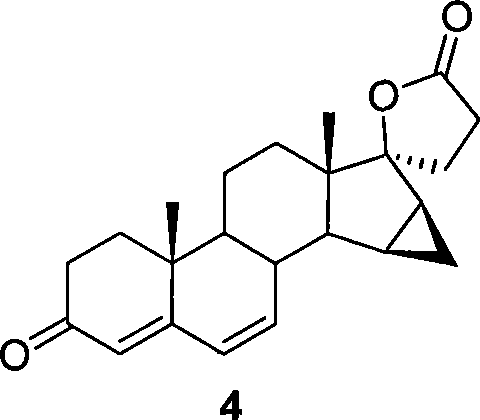
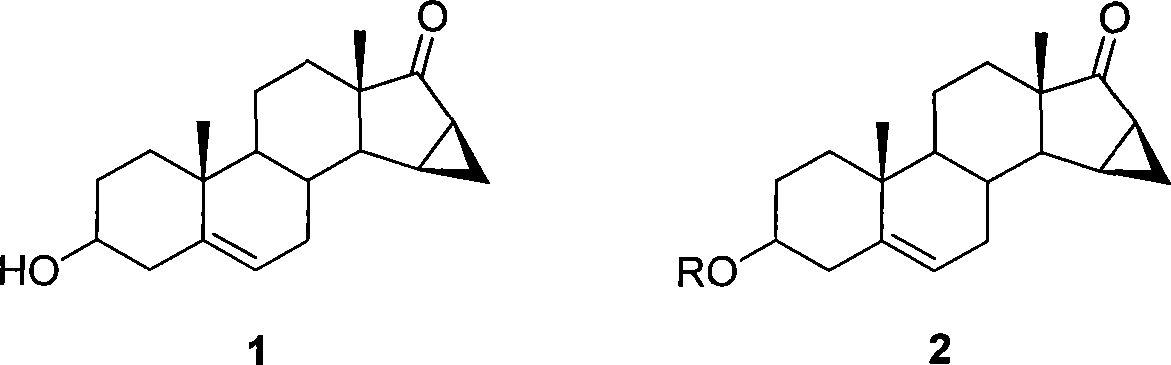Method for preparing drospiroenonand intermediate
A drospirenone and compound technology, applied in the direction of steroids, organic chemistry, etc., can solve the problem of high toxicity of chromium oxidant, and achieve the effect of reducing toxicity and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of 3-trimethylsilyloxyandrost-5-ene-15β,16β-methylene-17-one-step i
[0050]5 g of triethylamine was added to a solution of 10 g of 3-hydroxyandrost-5-ene-15β,16β-methylene-17-one and 200 mL of dichloromethane. At 0°C, 5.5 g of trimethylchlorosilane was added dropwise into the reaction solution. Stir at room temperature for 3 hours. Then pour the reaction solution into 100mL water, let it stand still, and separate the water layer. The organic layer was washed with 100 mL of water and 100 mL of saturated brine, and dried over anhydrous magnesium sulfate. After spin-drying, 12 g of a light yellow solid was obtained with a yield of 97.7%. The plies showed essentially no impurities. TLC: ethyl acetate: petroleum ether = 1:1
[0051] 1 H-NMR (400MHz, CDCl 3 )δ: 0.11(s, 6H), 0.97(s, 3H), 1.06(s, 3H), 2.23-1.05(m, 19H), 3.43(m, 1H), 5.40(d, 1H)
Embodiment 2
[0053] Preparation of 15β,16β-methylene-3β-hydroxy-17α-pregna-17β-hydroxy-21-hydroxymethyl-5-ene-step ii
[0054] Under the protection of nitrogen, 1.5 g of metallic lithium was added into 100 mL of tetrahydrofuran. At 0°C, a solution of 9 g of 3-trimethylsiloxyandrost-5-ene-15β,16β-methylene-17-one and 100 mL of tetrahydrofuran was added to the above solution. After 30 minutes, 16 g of chloropropyl trimethylsilyl ether was added dropwise to the reaction solution, keeping the temperature at 0°C. The reaction solution was stirred overnight at room temperature.
[0055] At 0°C, 8 mL of methanol was added to the reaction solution and stirred overnight. The reaction solution was poured into 300 mL of water, stirred for 1 hour and then filtered. The filter cake was washed with water until neutral and dried to obtain 7.08 g of off-white solid, with a yield of 81.3%. The plies showed essentially no impurities. TLC: dichloromethane: methanol = 4:1
Embodiment 3
[0057] Preparation of 15β,16β-methylene-3β-hydroxy-17α-pregna-17β-hydroxy-21-hydroxymethyl-5-ene-step ii
[0058] Under nitrogen protection, at 0°C, add 0.02 g of lithium metal to a solution of 0.124 g of 3-trimethylsilyloxyandrost-5-ene-15β, 16β-methylene-17-one and 5 mL of tetrahydrofuran , and then added 0.222 g of chloropropyltrimethylsilyl ether. The reaction solution was stirred overnight at room temperature.
[0059] Add 5 mL of methanol and stir overnight. Add 10 mL of water, stir for 1 hour and then filter. The filter cake is washed with water until neutral, and dried to obtain 0.1 g of off-white solid with a yield of 83.4%. The plies showed essentially no impurities. TLC: dichloromethane: methanol = 4:1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com