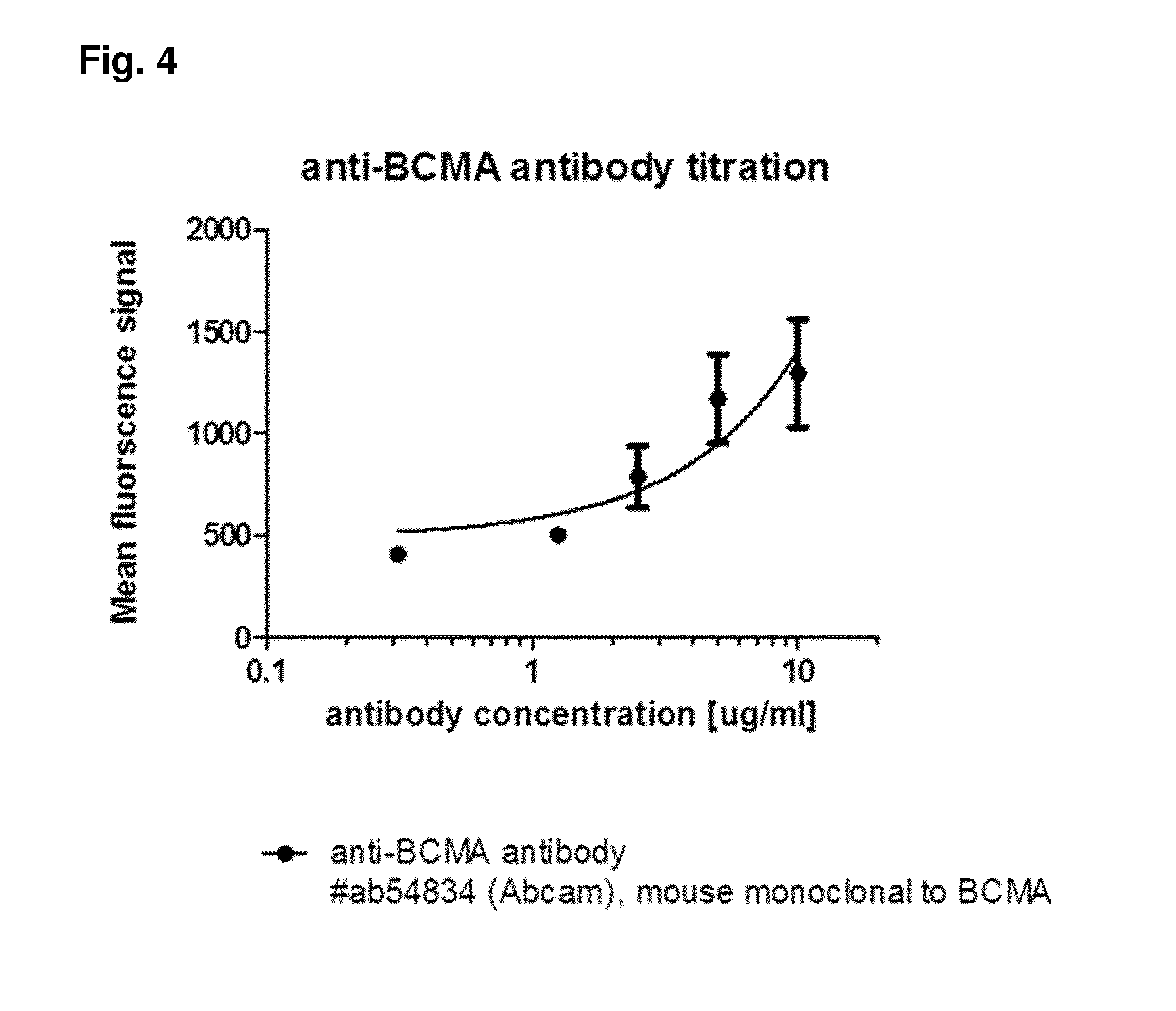
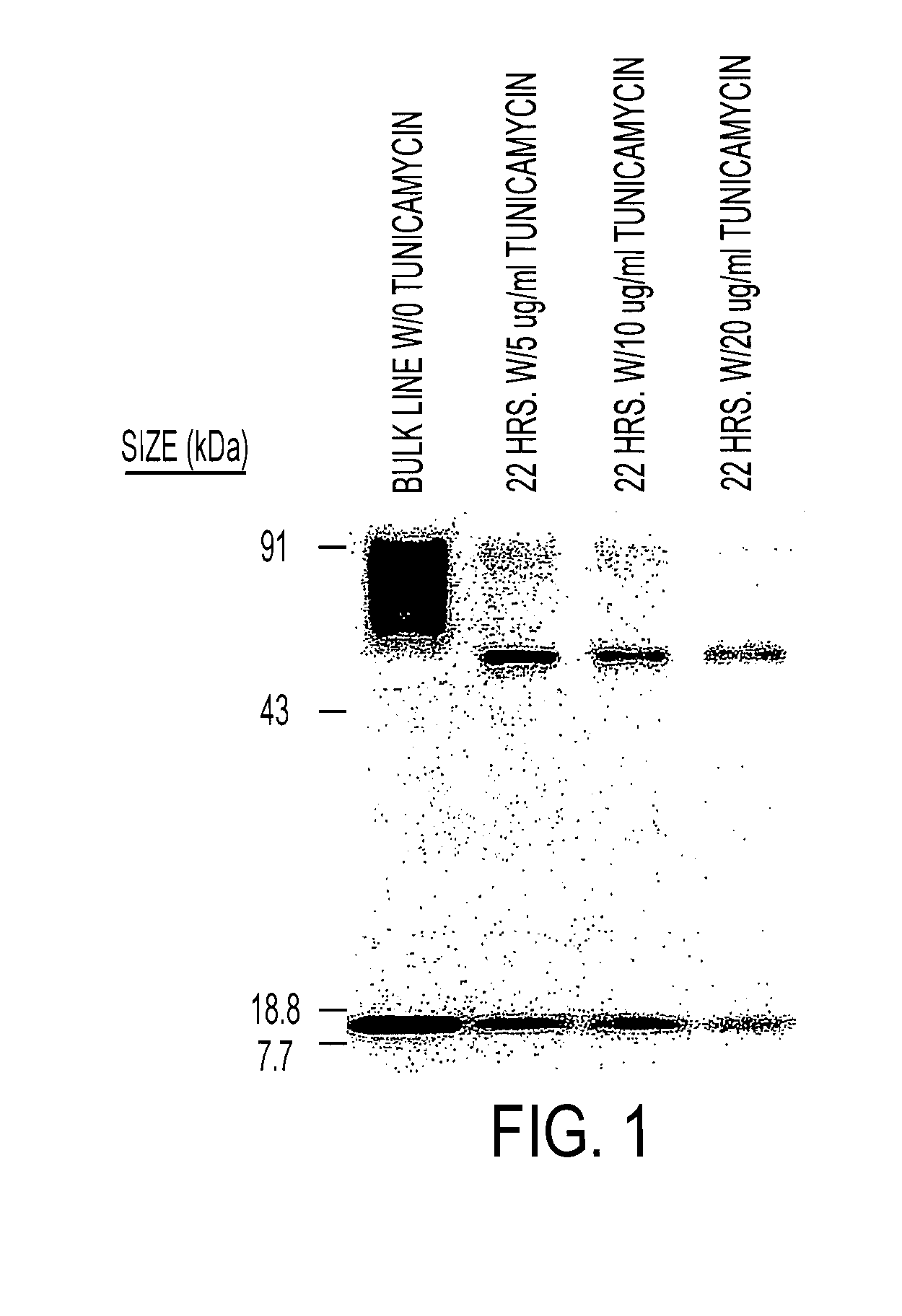
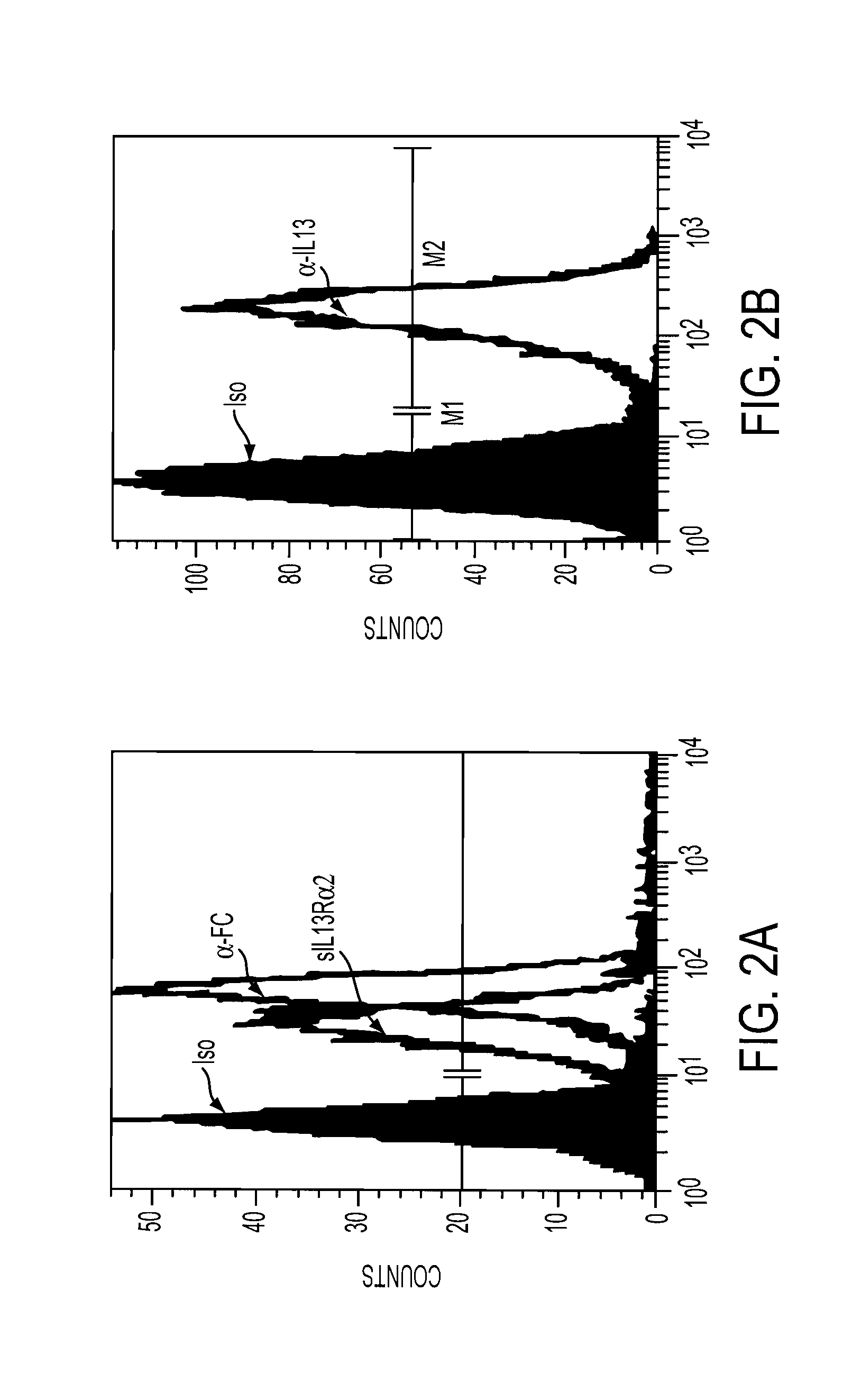
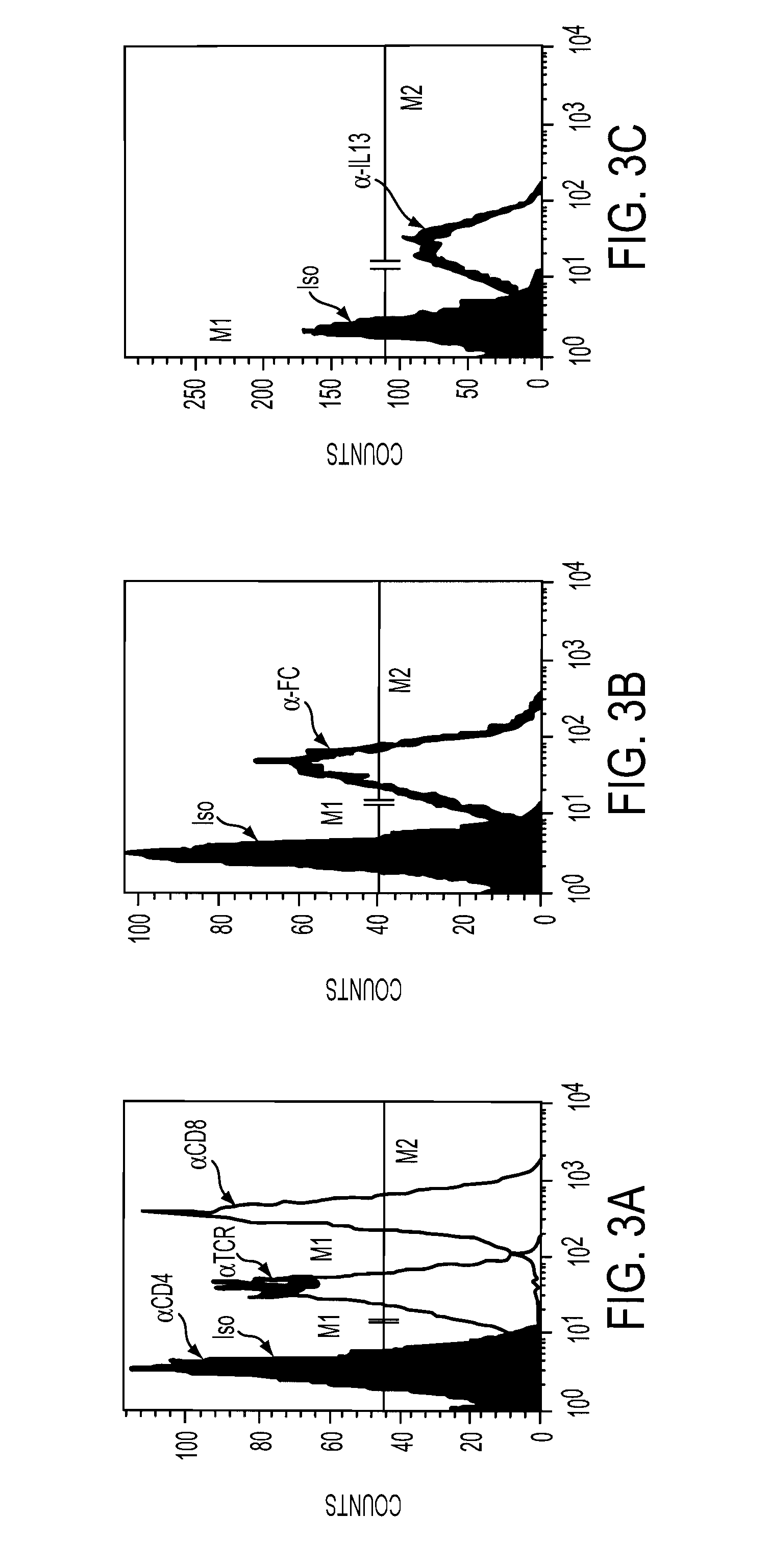
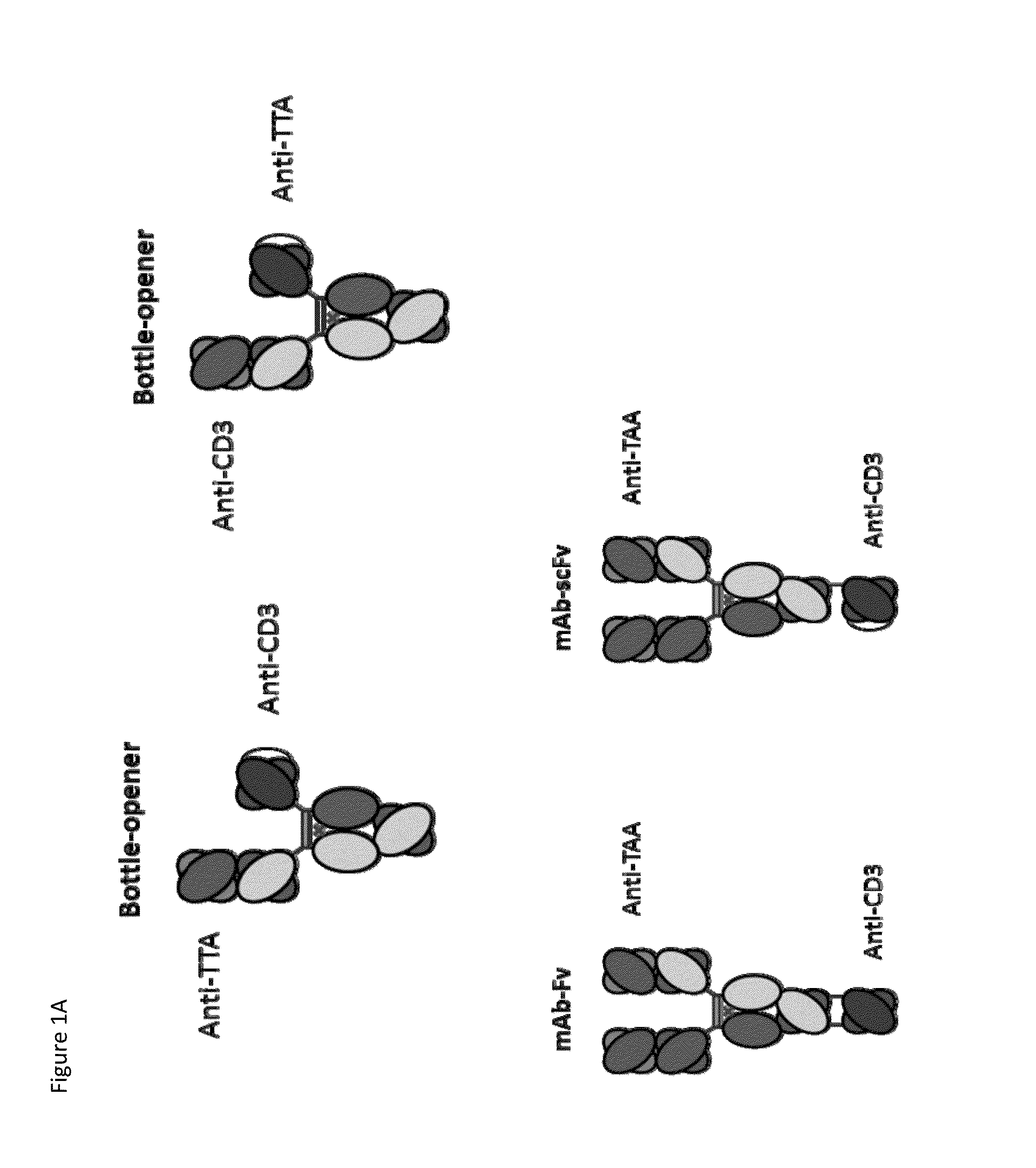
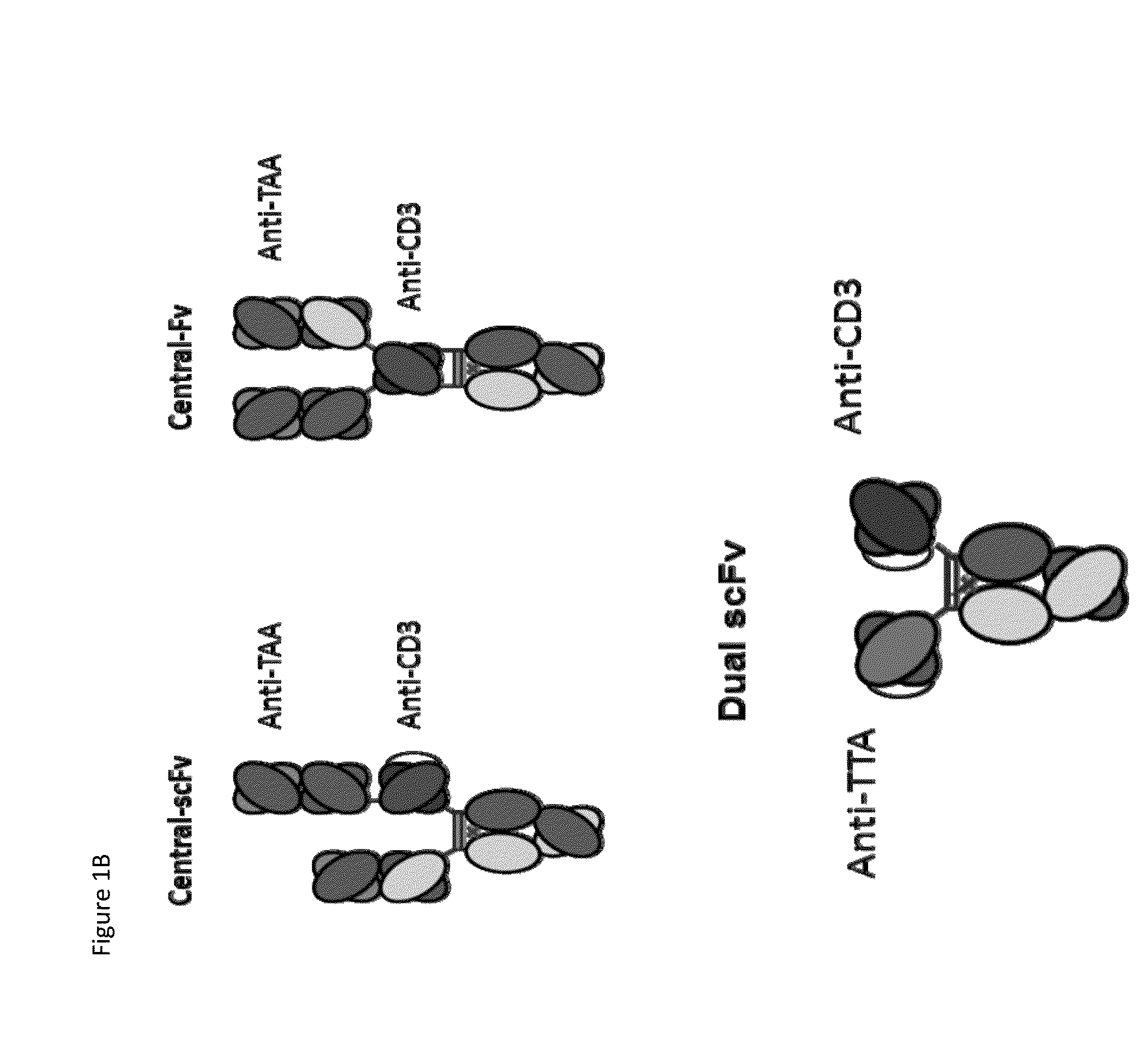
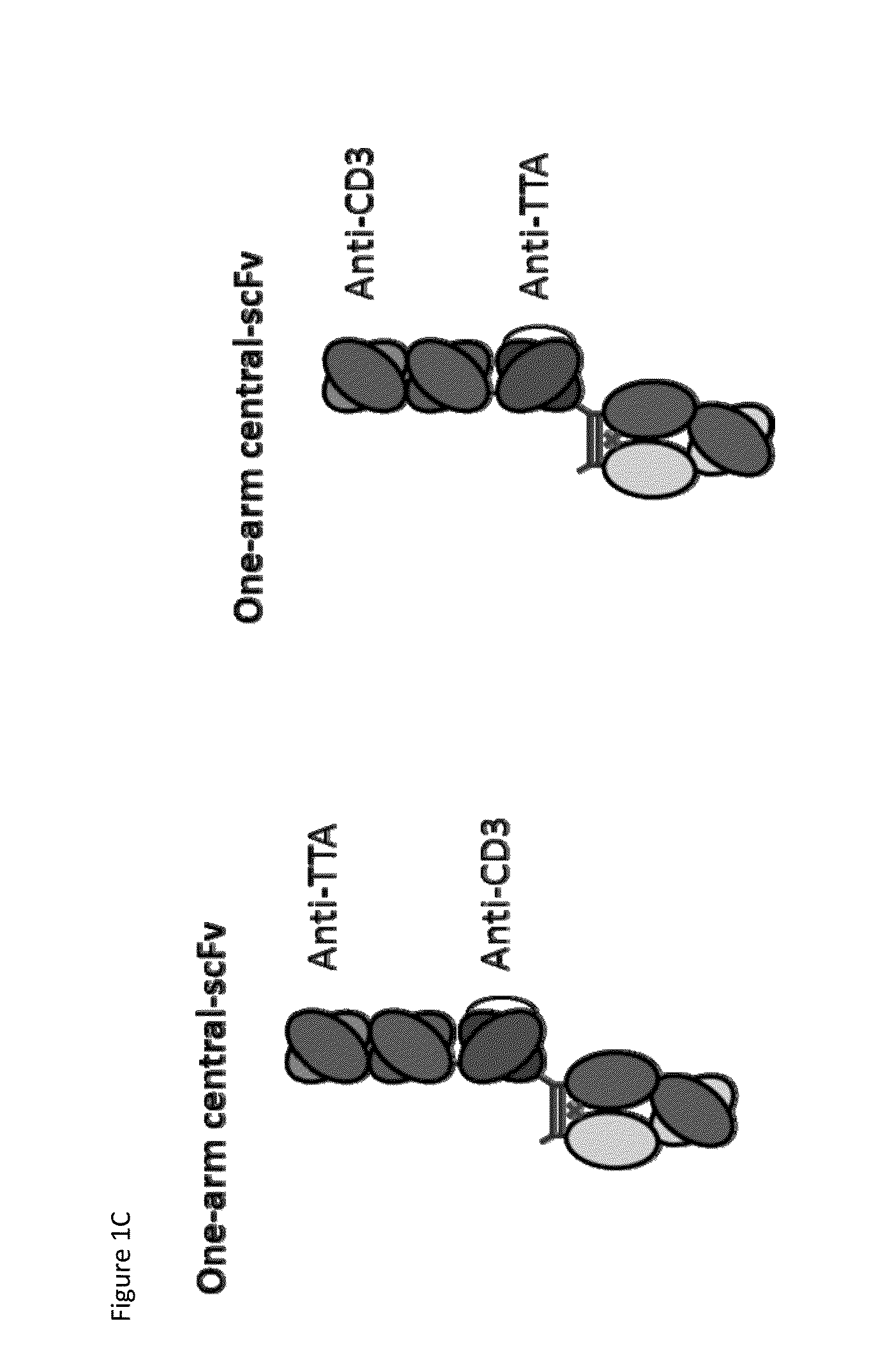
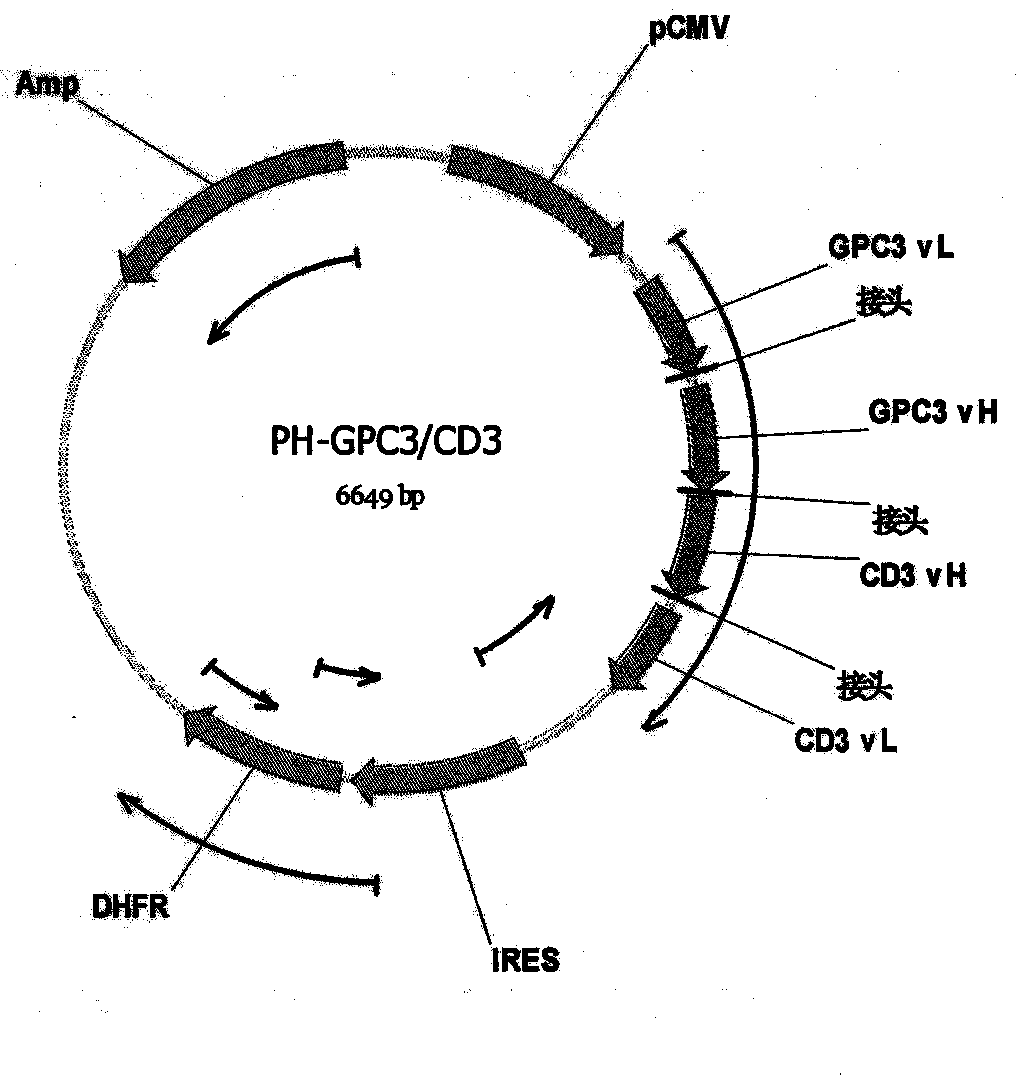
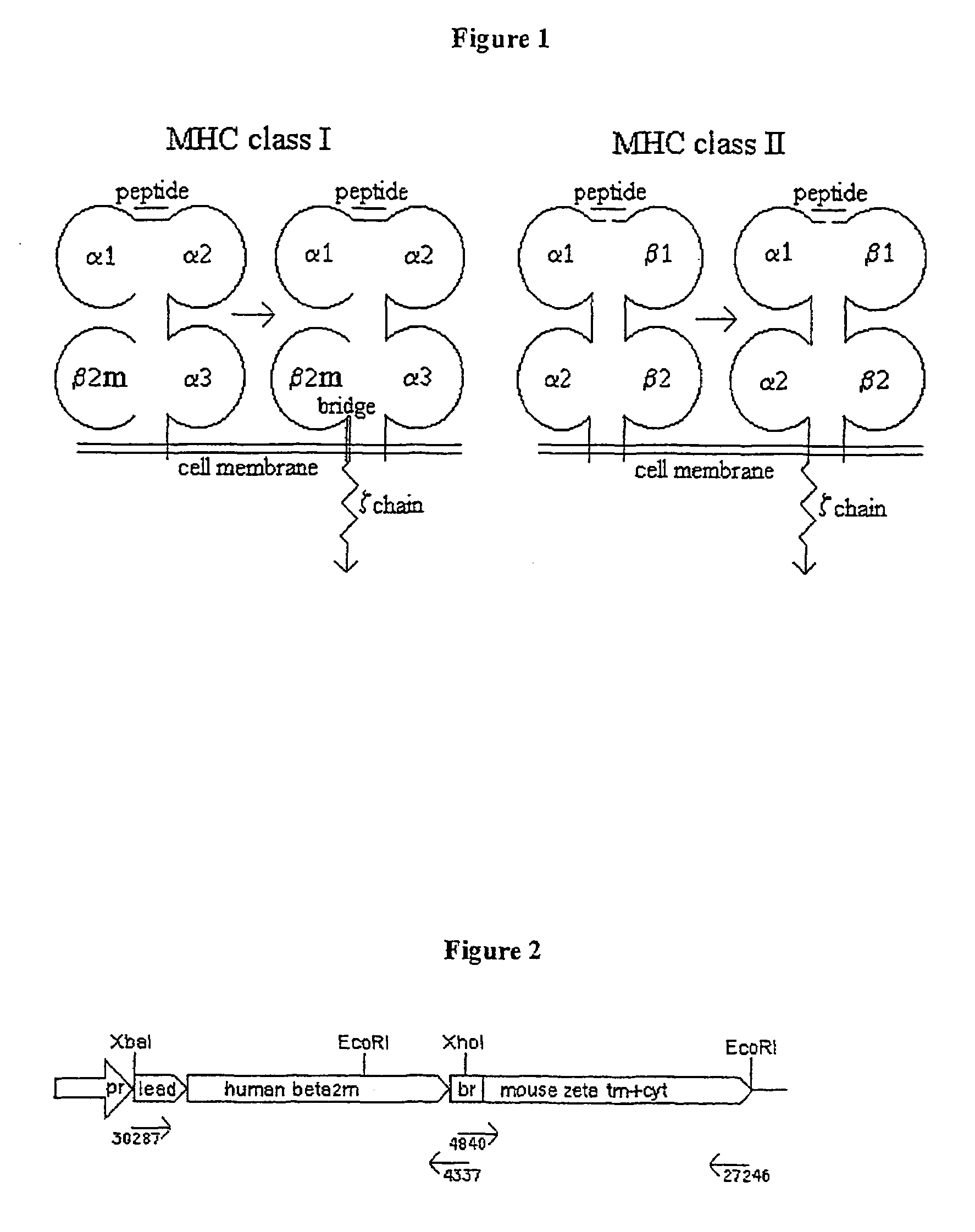
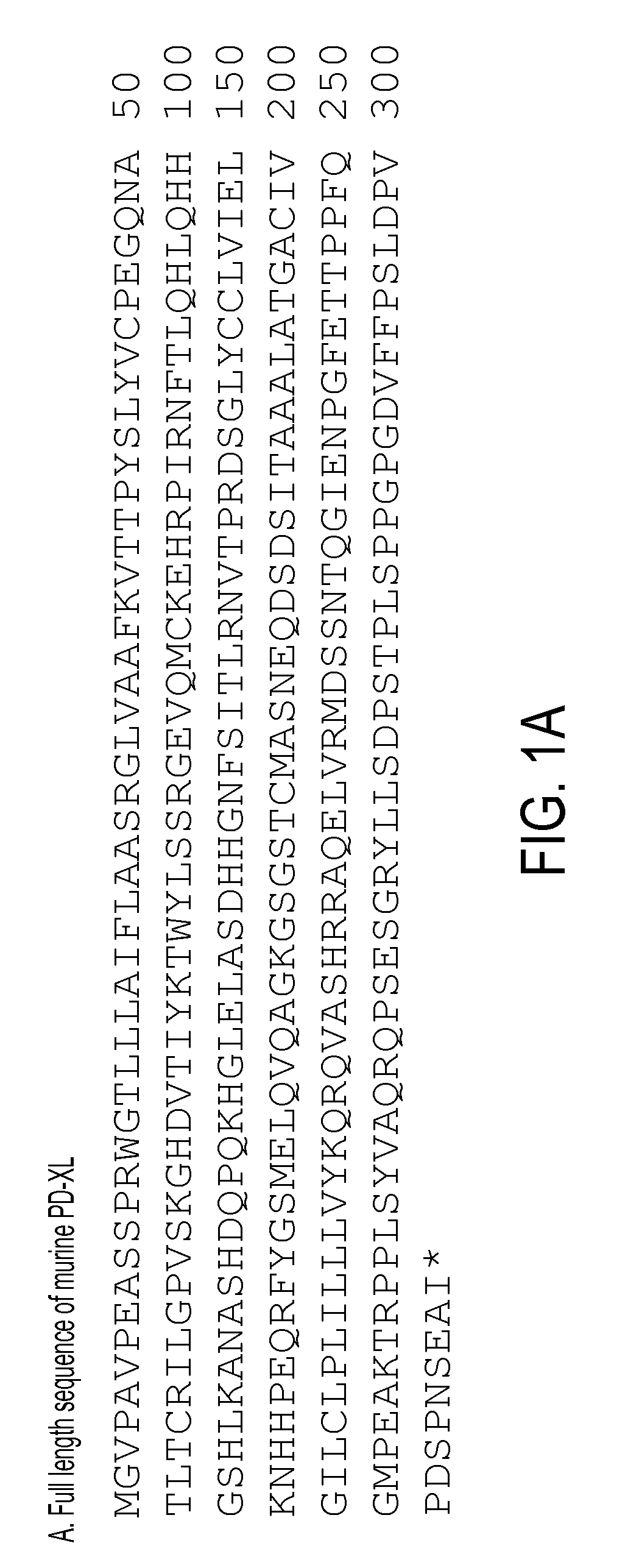
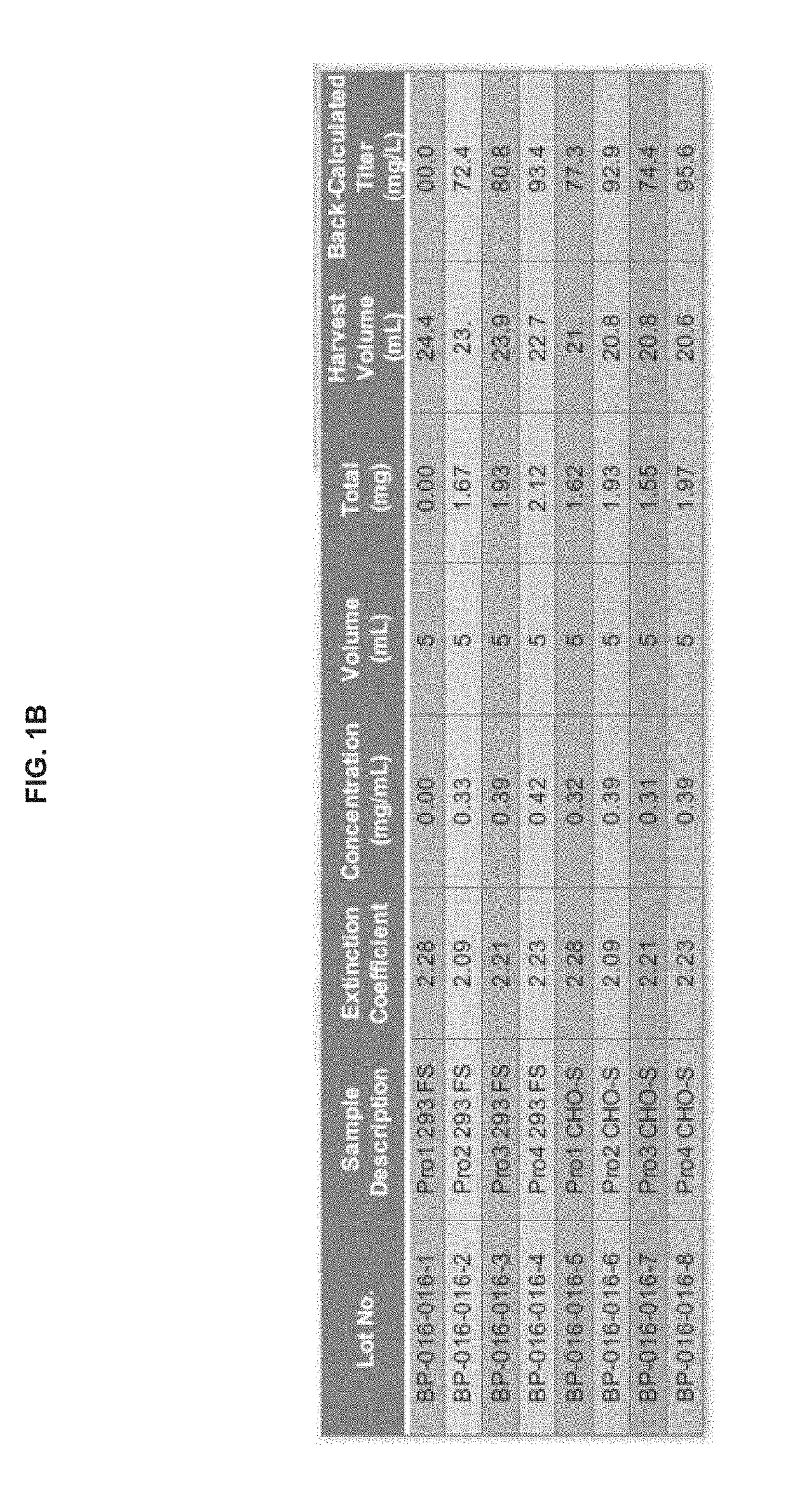
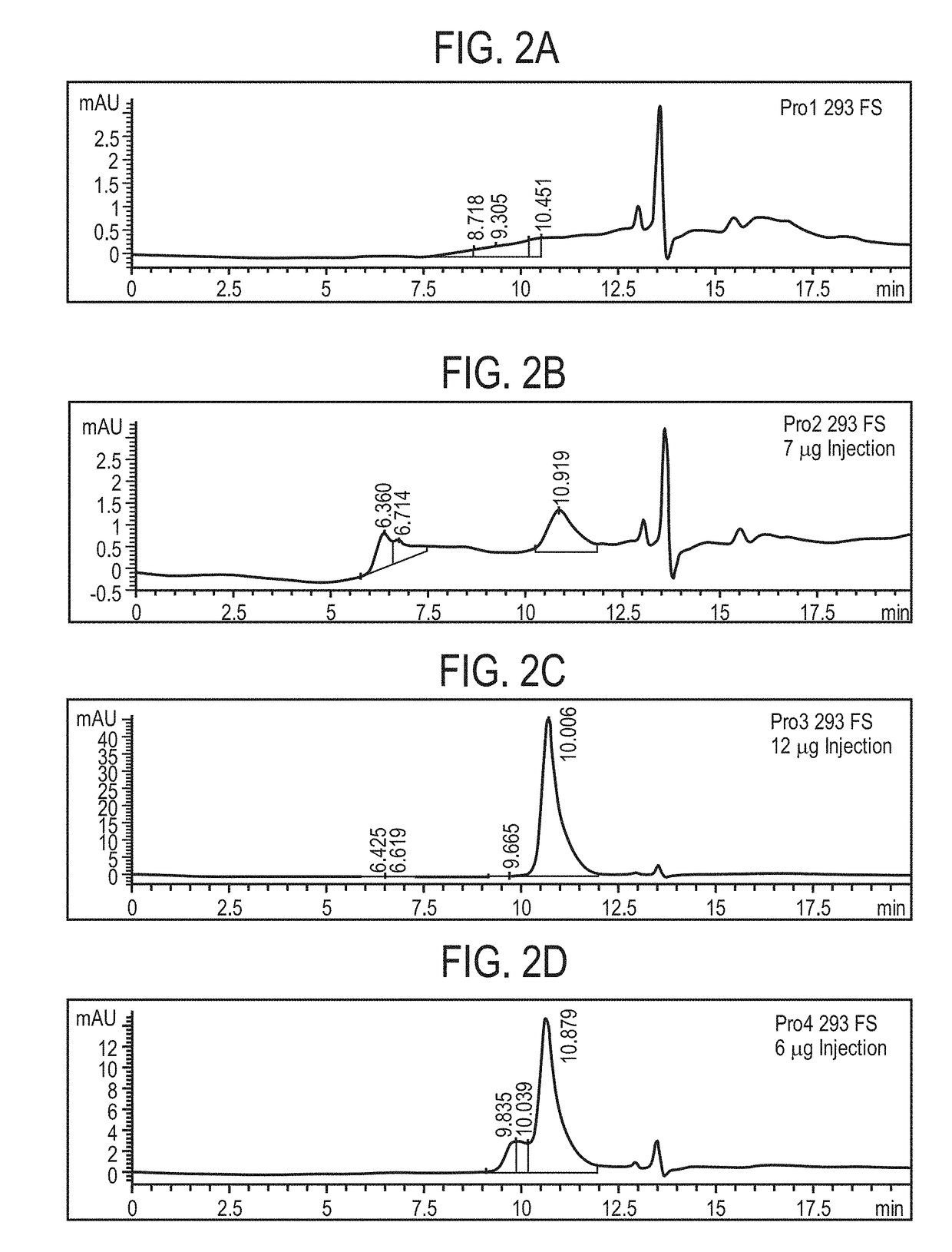
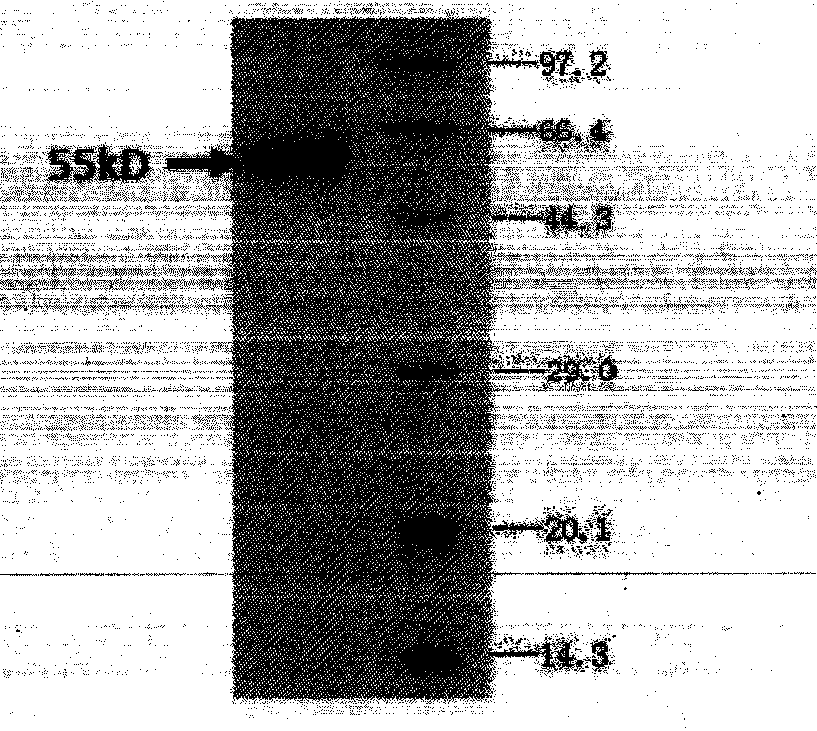Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
782 results about "CD3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
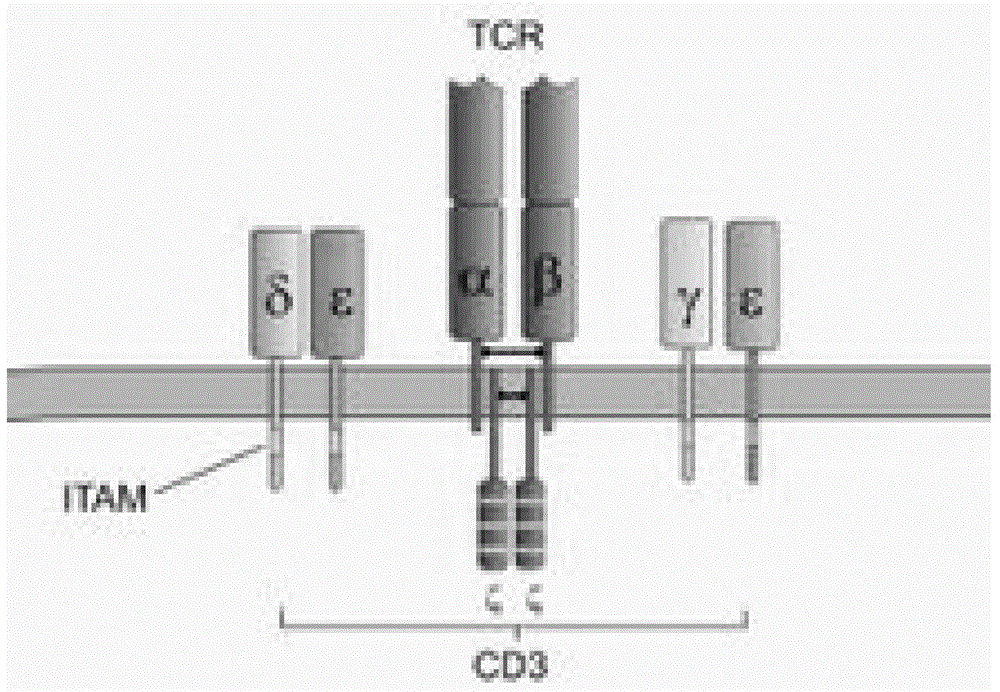
In immunology, the CD3 (cluster of differentiation 3) T cell co-receptor helps to activate both the cytotoxic T cell (CD8+ naive T cells) and also T helper cells (CD4+ naive T cells). It consists of a protein complex and is composed of four distinct chains. In mammals, the complex contains a CD3γ chain, a CD3δ chain, and two CD3ε chains. These chains associate with the T-cell receptor (TCR) and the ζ-chain (zeta-chain) to generate an activation signal in T lymphocytes. The TCR, ζ-chain, and CD3 molecules together constitute the TCR complex.
Cyclic single-chain trispecific antibody
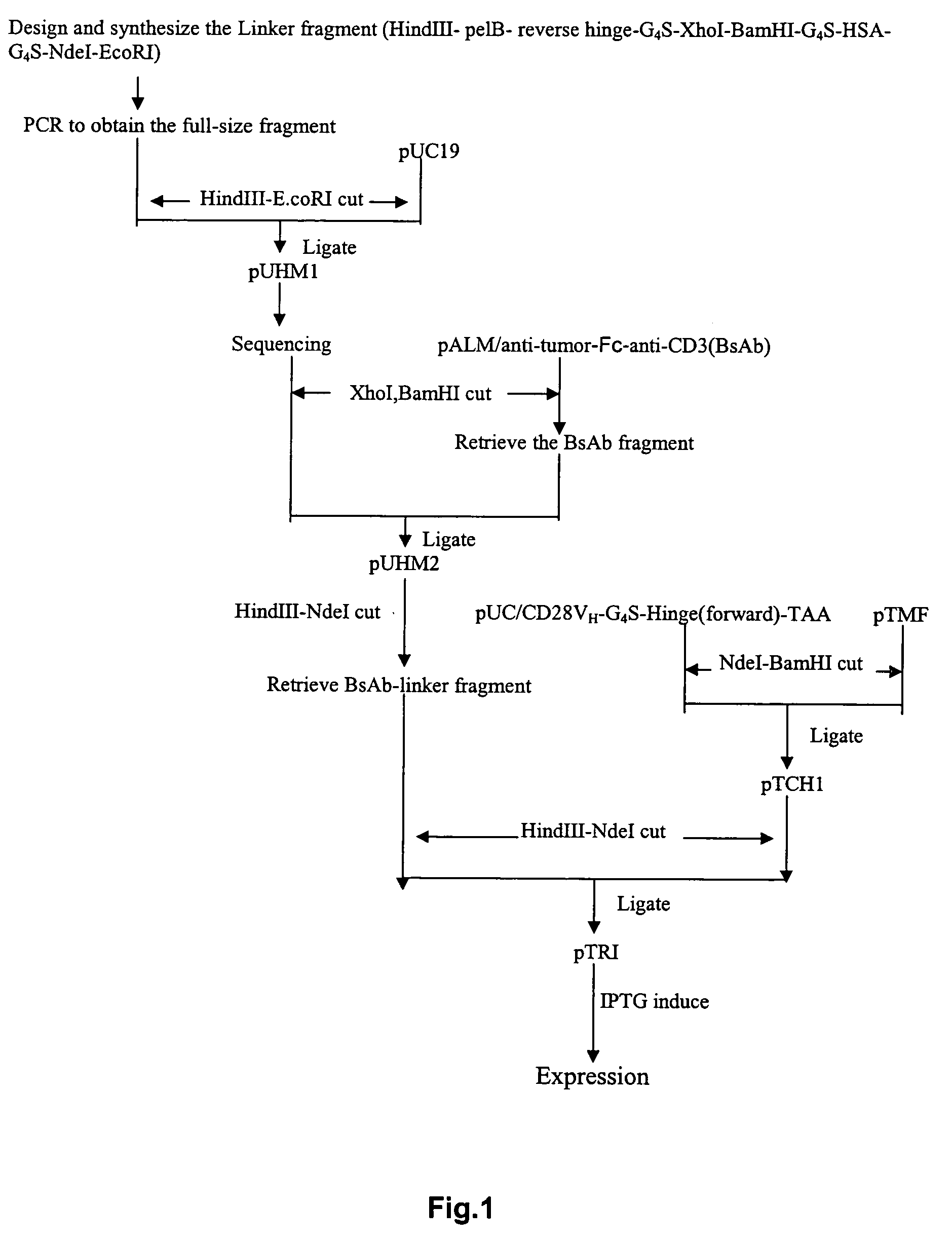
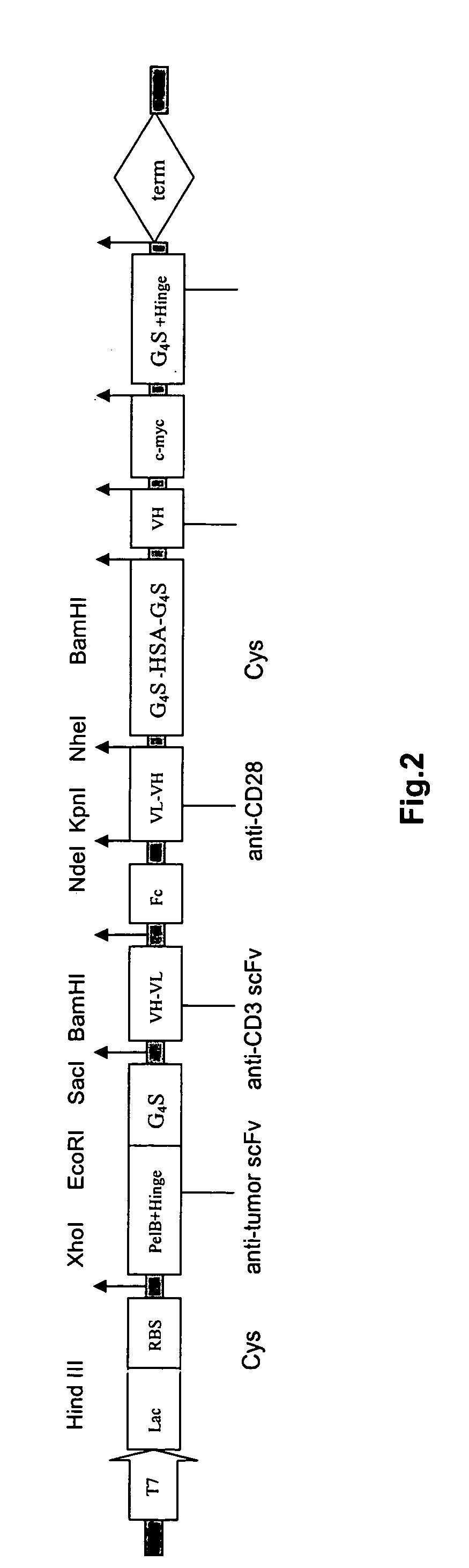
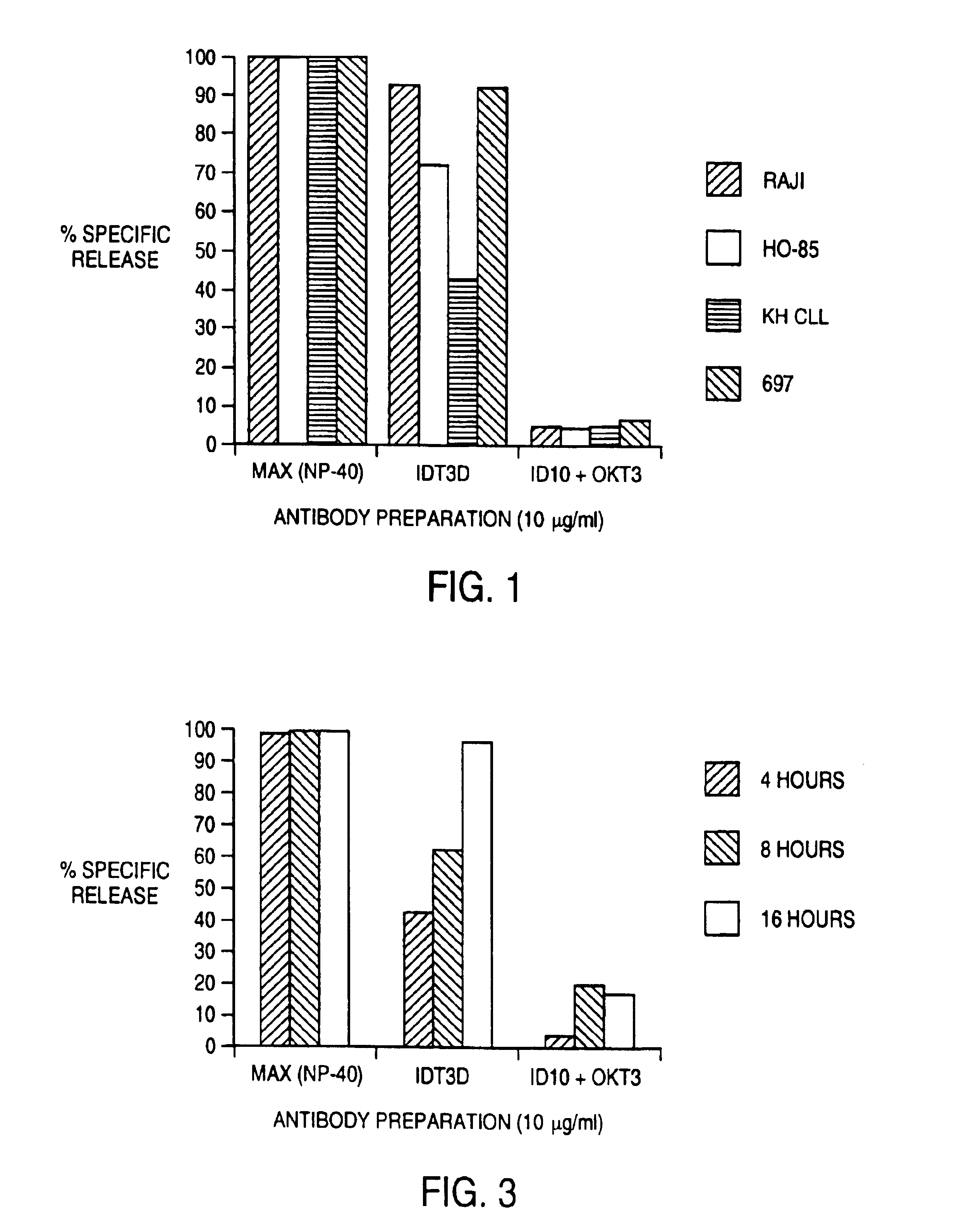
The invention provides a cyclic single-chain trispecific antibody against human tumor. It comprises three parts. The first part is an anti-tumor Fab antibody, an anti-tumor single-domain antibody or an scFv. The second part is a reshaped Fab antibody against human CD3, a reshaped single-domain antibody against human CD3 or a reshaped scFv against human CD3. The third part is a reshaped Fab antibody against human CD28, a reshaped single-domain antibody against human CD28 or a reshaped scFv against human CD28. The present invention also offers the DNA sequence coding for this trispecific antibody, expression vectors containing this DNA sequence and host cells (E. coli) containing the vectors.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Methods and Antibody Compositions for Tumor Treatment
ActiveUS20150266966A1Decreased killing of T-cellsReduce the burden onImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD20
The present invention provides bispecific antibodies that bind to CD3 and tumor antigens and methods of using the same. According to certain embodiments, the bispecific antibodies of the invention exhibit reduced effector functions and have a unique binding profile with regard to Fcγ receptors. The bispecific antibodies are engineered to efficiently induce T cell-mediated killing of tumor cells. According to certain embodiments, the present invention provides bispecific antigen-binding molecules comprising a first antigen-binding domain that specifically binds human CD3, a second antigen-binding molecule that specifically binds human CD20, and an Fc domain that binds Fcγ receptors with a specific binding pattern. In certain embodiments, the bispecific antigen-binding molecules of the present invention are capable of inhibiting the growth of B-cell or melanoma tumors expressing CD20. The bispecific antibodies of the invention are useful for the treatment of various cancers as well as other CD20-related diseases and disorders.
Owner:REGENERON PHARM INC
Humanized antibodies against CD3
Owner:ABBOTT BIOTHERAPEUTICS CORP
Bispecific antibodies against cd3 and bcma
Owner:BRISTOL MYERS SQUIBB CO
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Heterodimeric antibodies that bind cd3 and tumor antigens
ActiveUS20160229924A1Increase pI differenceHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsTumour-associated antigenTumor antigen
Owner:XENCOR
Bispecific antibody aiming at phosphatidylinositols protein polysaccharide-3 and T cell antigen
InactiveCN103833852ABacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenNucleotide
The first aspect of the invention relates to a bispecific antibody, which comprises a first functional domain for specific identification of phosphatidylinositol protein polysaccharide-3, a second domain for specific identification of human T cell antigen CD3, and a connection for connecting the functional domains. The second aspect of the invention relates to a nucleotide sequence encoding the above antibody. The third aspect of the invention relates to a carrier containing the above nucleotide sequence, and includes an expressive vector. The fourth aspect of the invention relates to a eukaryotic or prokaryotic expression system containing the above carrier. The fifth aspect of the invention relates to application of the above antibody to preparation of medicament for treating or preventing tumor.
Owner:SHANGHAI INST OF ONCOLOGY
Genetically-engineered MHC molecules
The invention provides DNA molecules encoding a chimeric polypeptide comprising (a) a component of a MHC molecule capable of association on a cell surface with an endogenous MHC molecule component of the same class, and (b) an intracellular region of a signal transduction element capable of activating T cells. Component (a) may be a monomorphic component and is preferably beta 2-microglobulin, or a polymorphic class I or class II component. The signal transduction element (b) capable of activating T cells may be a component of T-cell receptor CD3, preferably the CD3 zeta (zeta) polypeptide, a B cell receptor polypeptide or an Fc receptor polypeptide. Immune cells such as a CTLs expressing said chimeric MHC molecules specifically eliminate or inactivate harmful T cells and are useful for treating graft rejection and autoimmune diseases.
Owner:GAVISH GALILEE BIO APPL
Regulatory t cell mediator proteins and uses thereof
ActiveUS20110027278A1Eliminate the effects ofPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellAllergy
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNF. Proteins, antibodies, activated T cells and methods for using the same are disclosed.In particular methods of using these proteins and compounds, preferably antibodies, which bind or modulate (agonize or antagonize) the activity of these proteins, as immune modulators and for the treatment of cancer, autoimmune disease, allergy, infection and inflammatory conditions, e.g. multiple sclerosis is disclosed
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
HUMANIZED AND AFFINITY MATURED ANTIBODIES TO FcRH5 AND METHODS OF USE
ActiveUS20160368985A1Enhance immune functionBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyT cell
The present invention relates to anti-FcRH5 antibodies, including anti-FcRH5 antibodies comprising an FcRH5 binding domain and a CD3 binding domain (e.g., FcRH5 T cell-dependent bispecific (TDB) antibodies), and methods of using the same.
Owner:F HOFFMANN LA ROCHE & CO AG
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20060067920A1Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingAutocrine paracrine
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
CD19 targeting chimeric antigen receptor and NKT cell, and preparation method thereof and applications thereof
ActiveCN105418765AEnhance specific killing activityProlong survival timePeptide/protein ingredientsGenetic material ingredientsAntigenHinge region
The present invention discloses a chimeric antigen receptor, a gene and a recombinant expression vector thereof, an engineered CD19 targeting NKT cell and applications thereof. The chimeric antigen receptor is CD19ScFv-CD8-CD137-CD3 zeta, and consists of a hinge region and a transmembrane region of the CD19ScFv and CD8, an intracellular signal structural domain of CD137, and an intracellular signal structural domain of CD3 zeta, and the the hinge region, the transmembrane region, the intracellular signal structural domain of CD137 and the intracellular signal structural domain of CD3 zeta are connected in series. When used to treat advanced stage CD19-positive B-cell acute lymphocytic leukemia, the NKT cell modified by the chimeric antigen receptor CD19ScFv-CD8-CD137-CD3 zeta provided by the present invention has good specific killing activity on leukemic cells, and has certain therapeutic effect on advanced stage CD19-positive B-cell acute lymphocytic leukemia patients who are repeatedly subjected to therapy such as radiotherapy, chemotherapy and symptomatic therapy by other drugs but cannot recover obviously.
Owner:CELLULAR BIOMEDICINE GRP SHANGHAI +1
Inducible binding proteins and methods of use
InactiveUS20180134789A1Specific activityImprove pharmacokineticsAntibody mimetics/scaffoldsAntiviralsDiseaseProteinase activity
Provided herein are conditionally activated polypeptide constructs comprising a protease-activated domain binding to CD3, at least one half-life extension domain, and two or more domains binding to one or more target antigens. Also provided are pharmaceutical compositions thereof, as well as nucleic acids, recombinant expression vectors and host cells for making such polypeptide constructs. Also disclosed are methods of using the disclosed polypeptide constructs in the prevention, and / or treatment diseases, conditions and disorders.
Owner:TAKEDA PHARMACEUTICALS CO LTD
CD3-binding molecules capable of binding to human and non-human CD3
CD3-binding molecules capable of binding to human and non-human CD3, and in particular to such molecules that are cross-reactive with CD3 of a non-human mammal (e.g., a cynomolgus monkey) are presented. Uses of such antibodies and antigen-binding fragments in the treatment of cancer, autoimmune and / or inflammatory diseases and other conditions are presented.
Owner:MACROGENICS INC
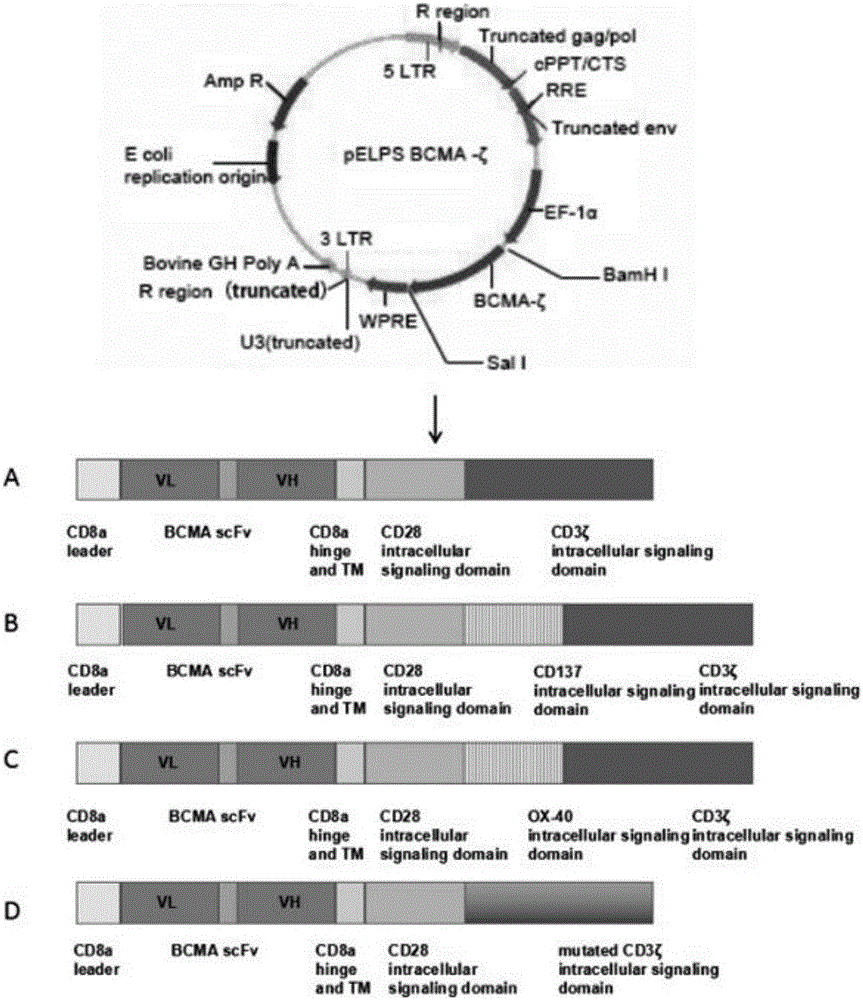
BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
InactiveCN105837693AImprove anti-apoptotic abilityImprove bindingAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen
The invention provides a BCMA-based (B cell maturation antigen-based) chimeric antigen receptor, comprising following cis-form cascade domains: CD8a leader region, scFv fragment BCMA scFv of anti-BCMA antibody, CD8a hinge region and transmembrane region, CD28 intracellular signal domain and CD3 Zeta intracellular signal domain; the CD3 Zeta intracellular signal domain is wild CD3 Zetaintracellular signal domain or mutant CD3 Zeta mut intracellular signal domain; the BCMA-based chimeric antigen receptor can enhance anti-apoptotic capability of T-cells and enhance CAR T-cell and antigen bonding and signal conduction; T-cells with the BCMA-based chimeric antigen receptor show good tumor targeting performance in in-vivo experiments, tumors diminish significantly after two weeks of dosage, and the T-cells have excellent therapeutic effect in vivo.
Owner:李斯文 +1
Stem Cell Populations and Methods of Use
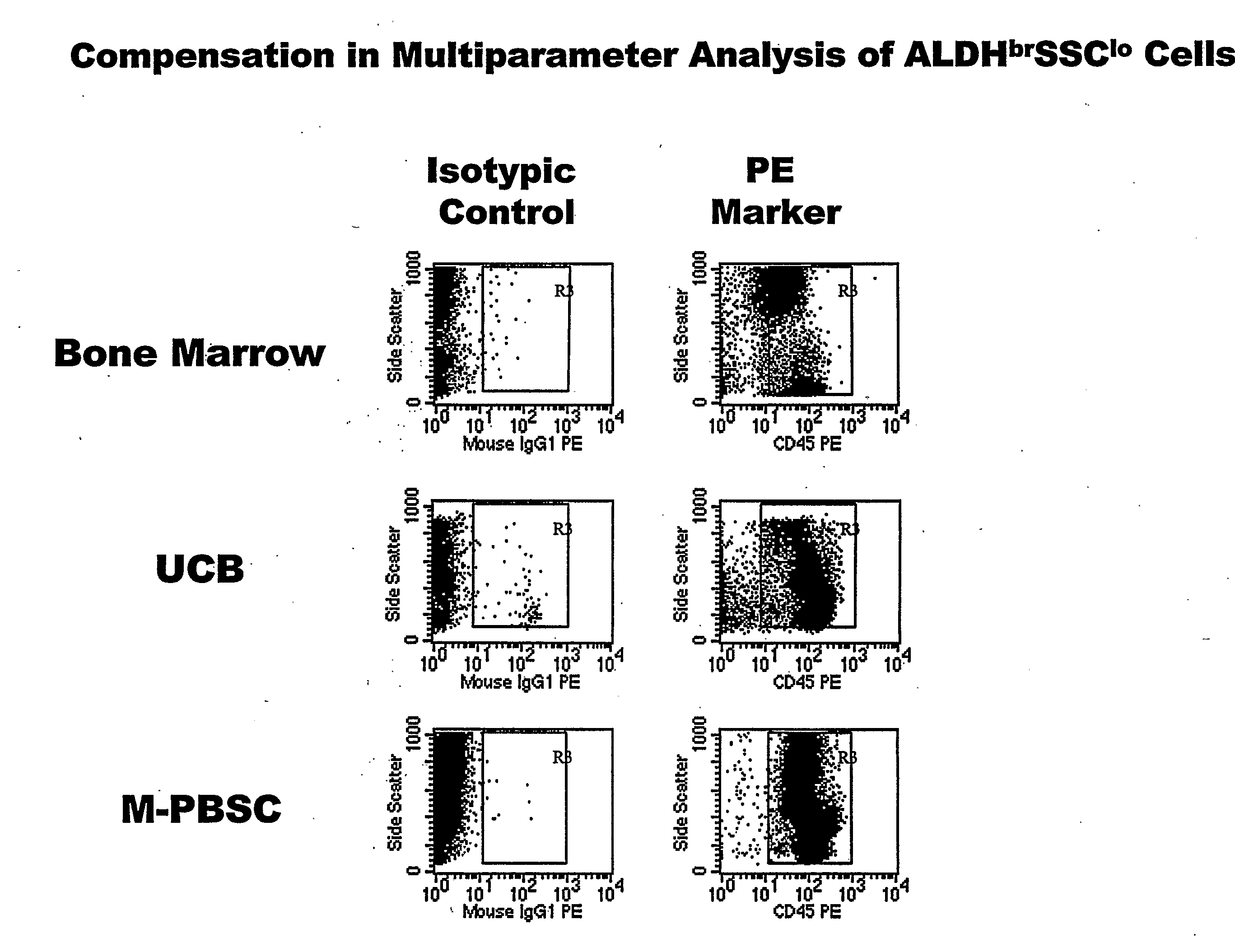
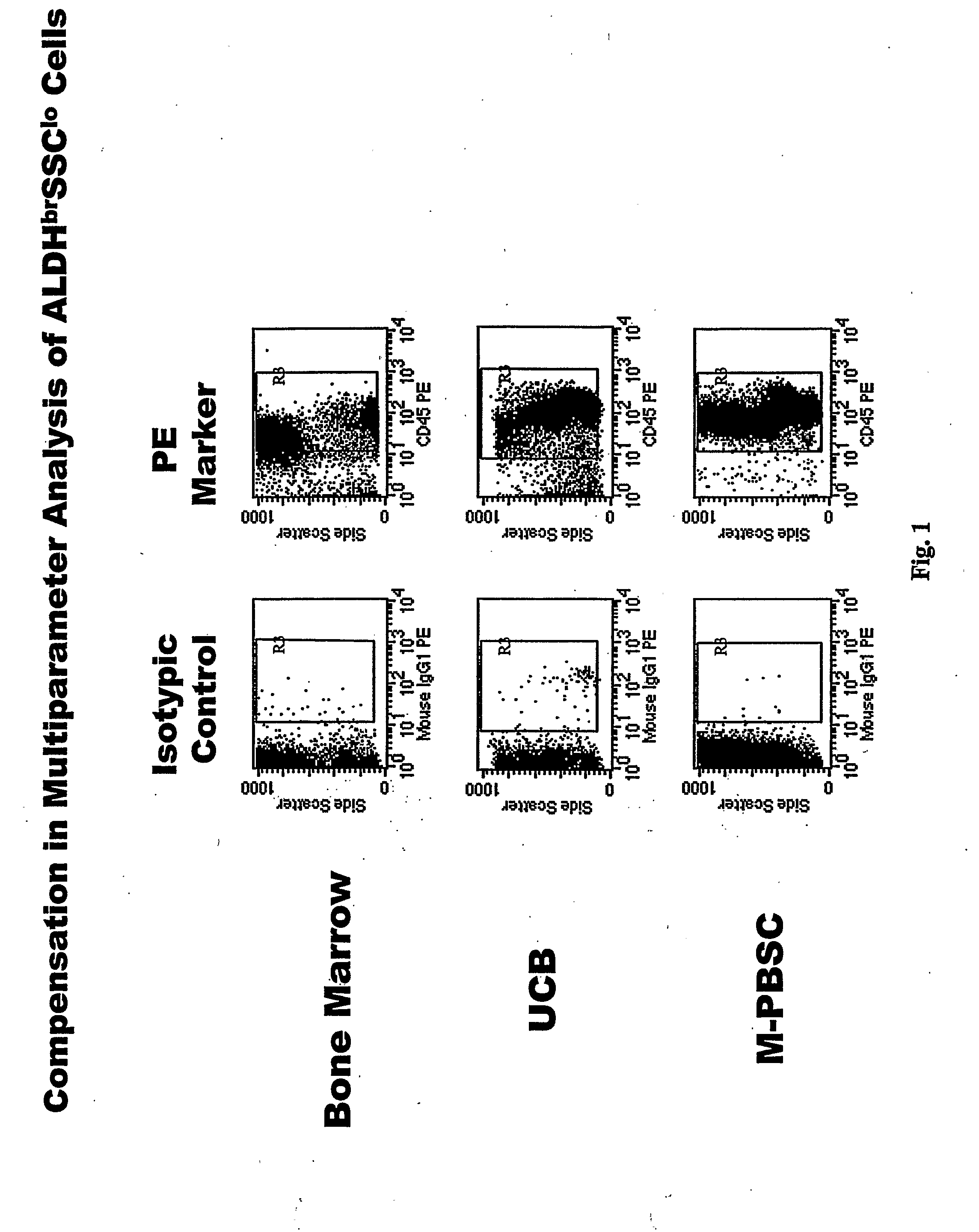
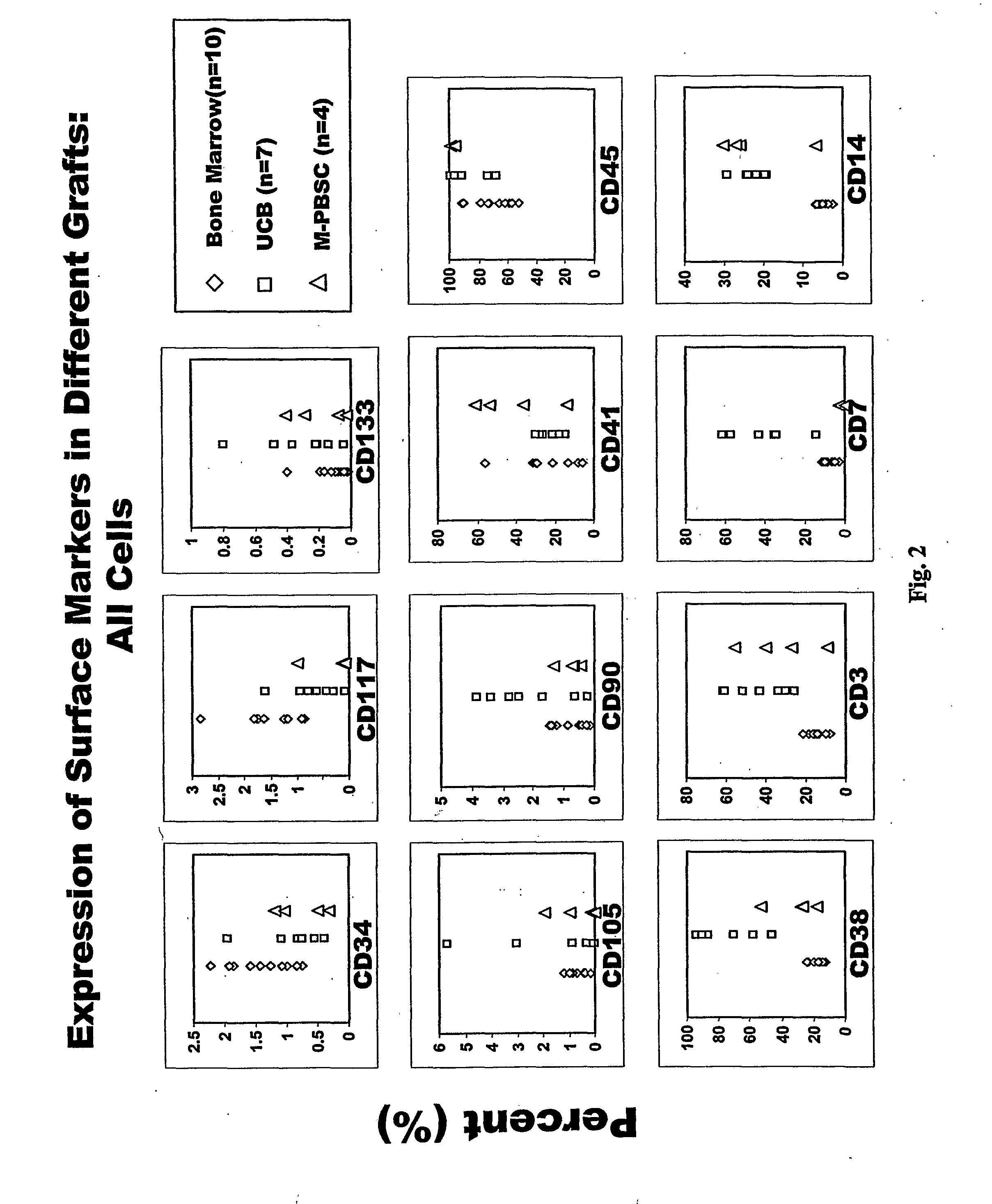
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
Preparation method and application of PD-1/CTLA-4 (programmed death-1/cytotoxic T lymphocyte antigen-4) bispecific antibody
InactiveCN105754990ABlock immunosuppressive signalingEnhance the function of killing cancer cellsHybrid immunoglobulinsAntibody ingredientsPolymerase chain reactionImmunosuppression
The invention discloses a preparation method and application of a PD-1 / CTLA-4 (programmed death-1 / cytotoxic T lymphocyte antigen-4) bispecific antibody. The preparation method comprises the following steps of using a single-chain antibody gene as a template and introducing disulfide bond into a variable region of the antibody by a particular high-efficiency amplification primer through PCR (polymerase chain reaction) and Overlap PCR through a gene engineering technique, so as to prepare a PD-1 / CTLA-4 bispecific antibody fragment; cloning the gene fragment to a chronic virus expression carrier, and transfecting 293T cells to package the virus; transducing a CIK (cytokine induced killer) cell, separating PBMC (peripheral blood mononuclear cell) from peripheral blood of a health person, using CD3 / CD28 magnetic beads to stimulate the growth of the T cell, and co-culturing the chronic virus and the T cell, so as to obtain a novel anti-tumor T cell which can block an immunosuppression signal of the tumor and tumor micro environment source, and enhance the function of killing cancer cells.
Owner:杨晶
Use of the cd2 signaling domain in second-generation chimeric antigen receptors
InactiveCN104136458AAvoid deathPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell expressing a CAR having an antigen binding domain, a transmembrane domain, a CD2 signaling domain, and a CD3 zeta signaling domain. The invention also includes incorporating CD2 into the CAR to alter the cytokine production of CAR-T cells in both negative and positive directions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
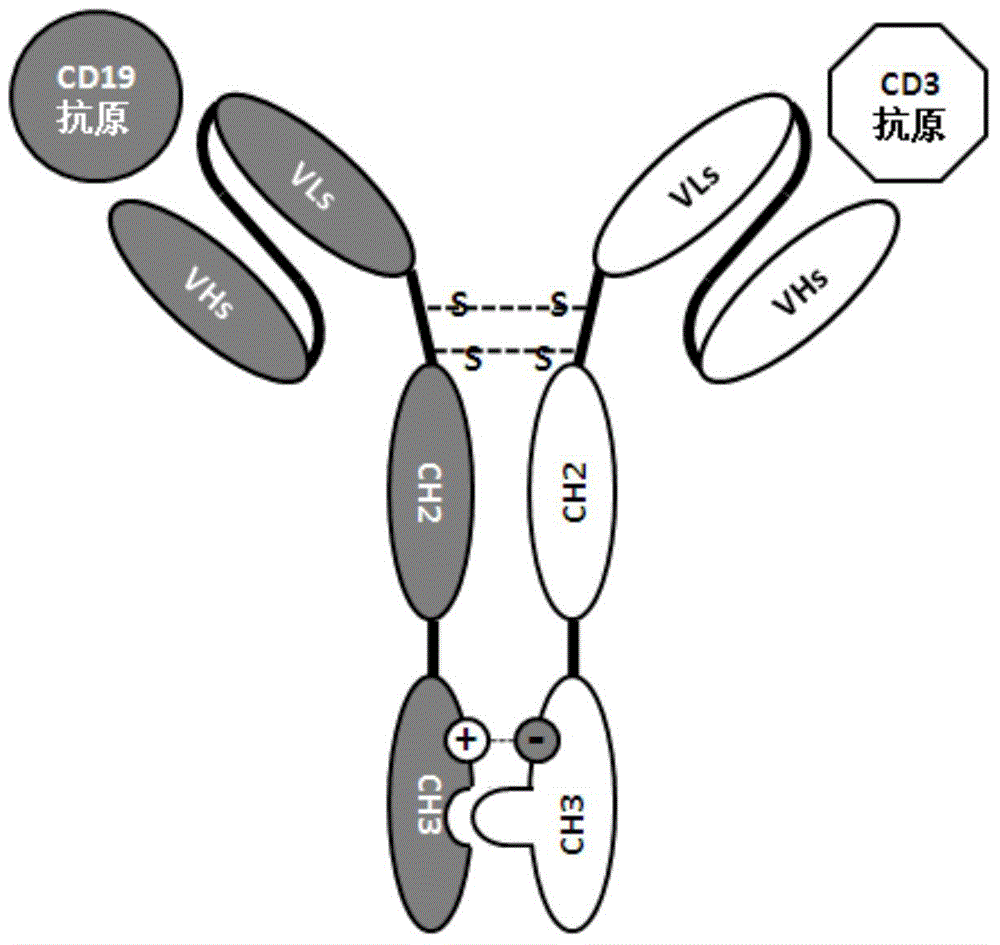
Construction and application of bispecific antibody CD19*CD3
ActiveCN104829726AIncrease lethalityIncreased immunotherapyHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsBispecific antibodyTumor cells
The invention provides a bispecific antibody which is composed of a single-chain unit A and a single-chain unit B, wherein the single-chain unit A, aiming to surface antigen CD19 of immune cells, has a specifically combining capability while the single-chain unit B, aiming to surface antigen CD3 of tumor cells, has a specifically combining capability. The single-chain unit A and the single-chain unit B both include a single-chain variable fragment (ScFv) fused with an Fc fragment. The application also provides a preparation of the bispecific antibody and medicinal applications of these antibodies.
Owner:WUHAN YZY BIOPHARMA CO LTD
Culture medium efficiently amplifying autologous NK cells and cultural method
The invention relates to a culture medium efficiently amplifying autologous NK cells and a culture method. The culture medium internally contains interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNFalpha) and CD3-antibody, can efficiently amplify and activate NK cells so as to obtain a large quantity of highly active immune cells with majority of NK cells to be transferred back to human bodies, and has remarkable curative effect in anti-tumor, anti-virus infection and immune adjustment related diseases. The culture medium efficiently amplifying autologous NK cells comprises the culture medium for cultivating cells and added factors added into the culture medium for cultivating cells, is used for cultivating and amplifying a large quantity of autologous activated NK cells, and is characterized in that the added factor comprises interleukin 2(IL-2), interleukin 15(IL-15), interleukin 7(IL-7), interleukin 12(IL-12), tumor necrosis factor alpha (TNF alpha) and CD3-antibody.
Owner:康思葆(北京)生物技术有限公司
Preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell
ActiveCN102154206AIncrease the number ofHigh activityMammal material medical ingredientsBlood/immune system cellsPhytohemagglutininsPeripheral blood mononuclear cell
The invention belongs to the technical field of cell culture in vitro, and particularly relates to a preparation method of high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The method comprises the following steps: collecting and separating peripheral blood mononuclear cell of a patient, eliminating CD4+CD25+Treg cell by means of Mini MACS (magnetic active cell sorting) method, and sorting to obtain CD3+, CD4+ and CD8+T cells; and putting the obtained cells into culture solution containing phytohemagglutinin (PHA), so that the PHA concentration in the suspension liquid is 100ng / ml, hatching for 24h under the culture condition of 5% CO2 at 37 DEG C, transferring the hatched suspension liquid into a cell culture bottle coated by CD3 monoclonal antibody (1mug / ml), adding IFN (interferon)-gamma (1000U / ml), adding IL (interleukin)-2(500U / ml) and IL (interleukin)-21(1000U / ml) after 48h, compensating sodium selenite-containing (0.005mg / L)cell culture after four days, and continuously culturing for 7-14 days to obtain the high-purity, high-multiplication capacity and high-cytotoxin activity CIK (cytokine induced kill) cell. The quantity, the activity and the purity of the CIK cell which is prepared by the method and amplified in vitro are improved, so that the antineoplastic function of the CIK is enhanced.
Owner:郑骏年
Chimeric antigen receptors (CAR) targeting hematologic malignancies, compositions and methods of use thereof
The present disclosure provides chimeric antigen receptor polypeptides having antigen recognition domains for CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens, and polynucleotides encoding for the same. The present disclosure also provides for engineered cells expressing the polynucleotide or polypeptides. In some embodiments, the disclosure provides methods for treating diseases associated with CD2, CD3, CD4, CD5, CD7, CD8, and CD52 antigens.
Owner:ICELL GENE THERAPEUTICS LLC +5
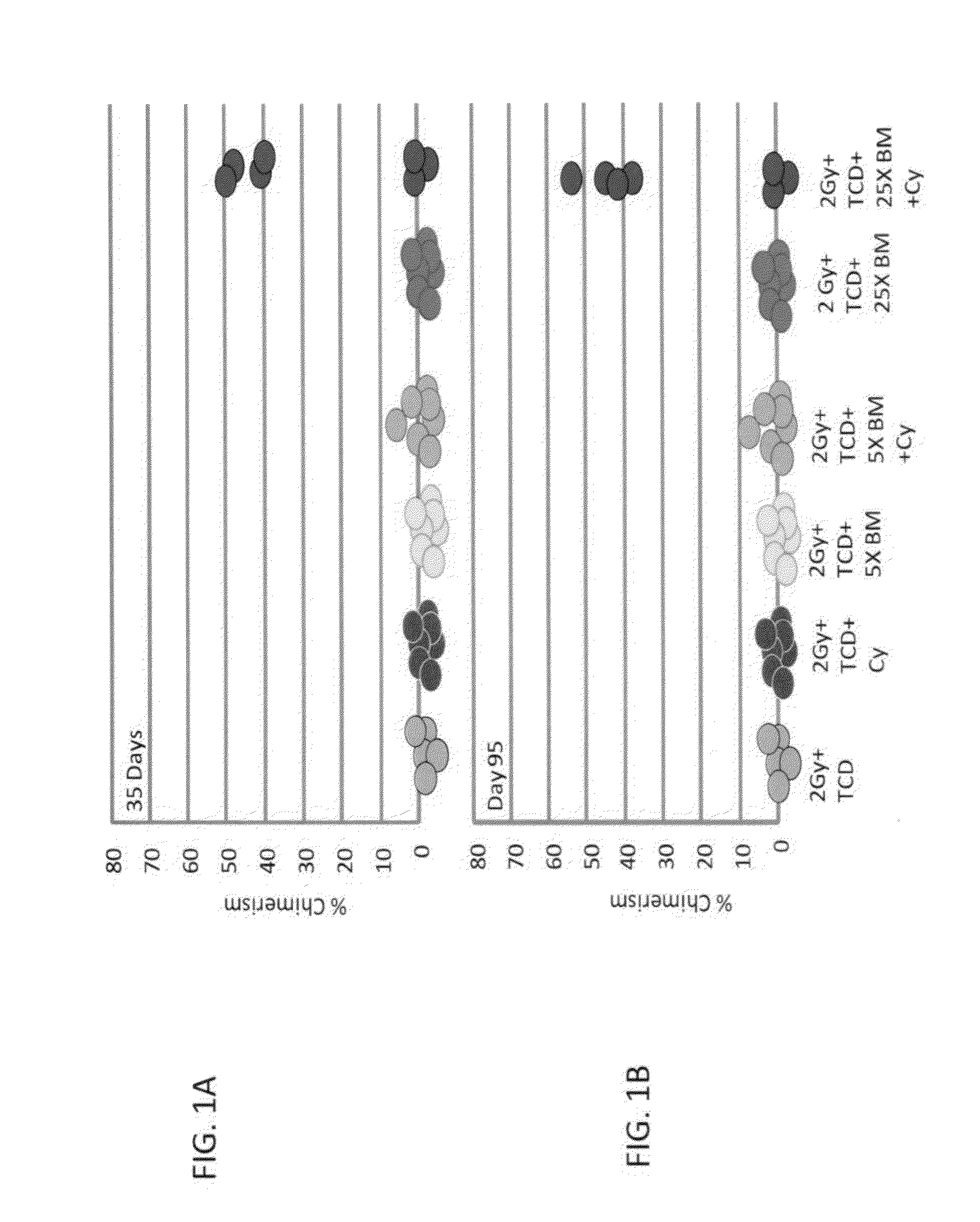
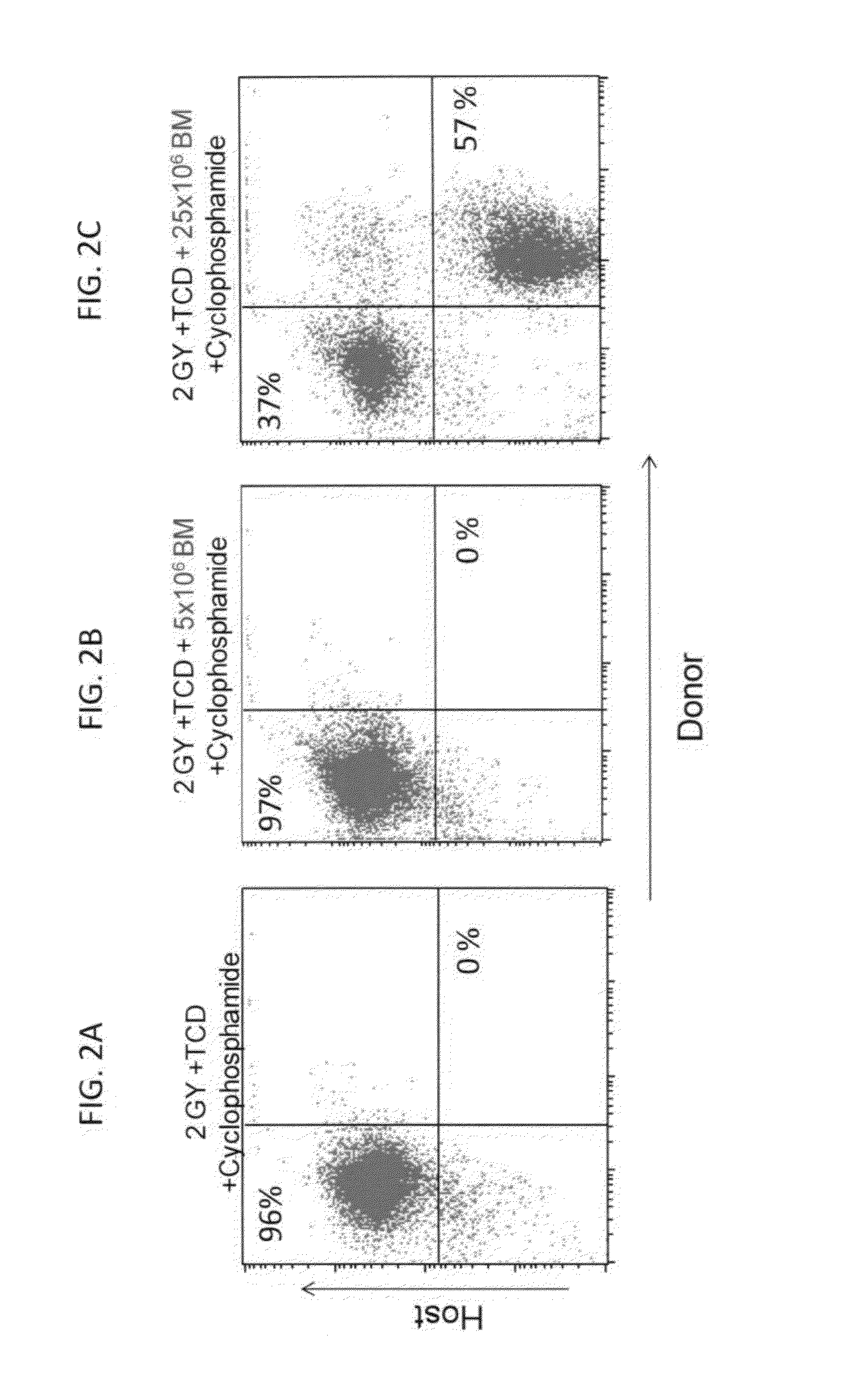
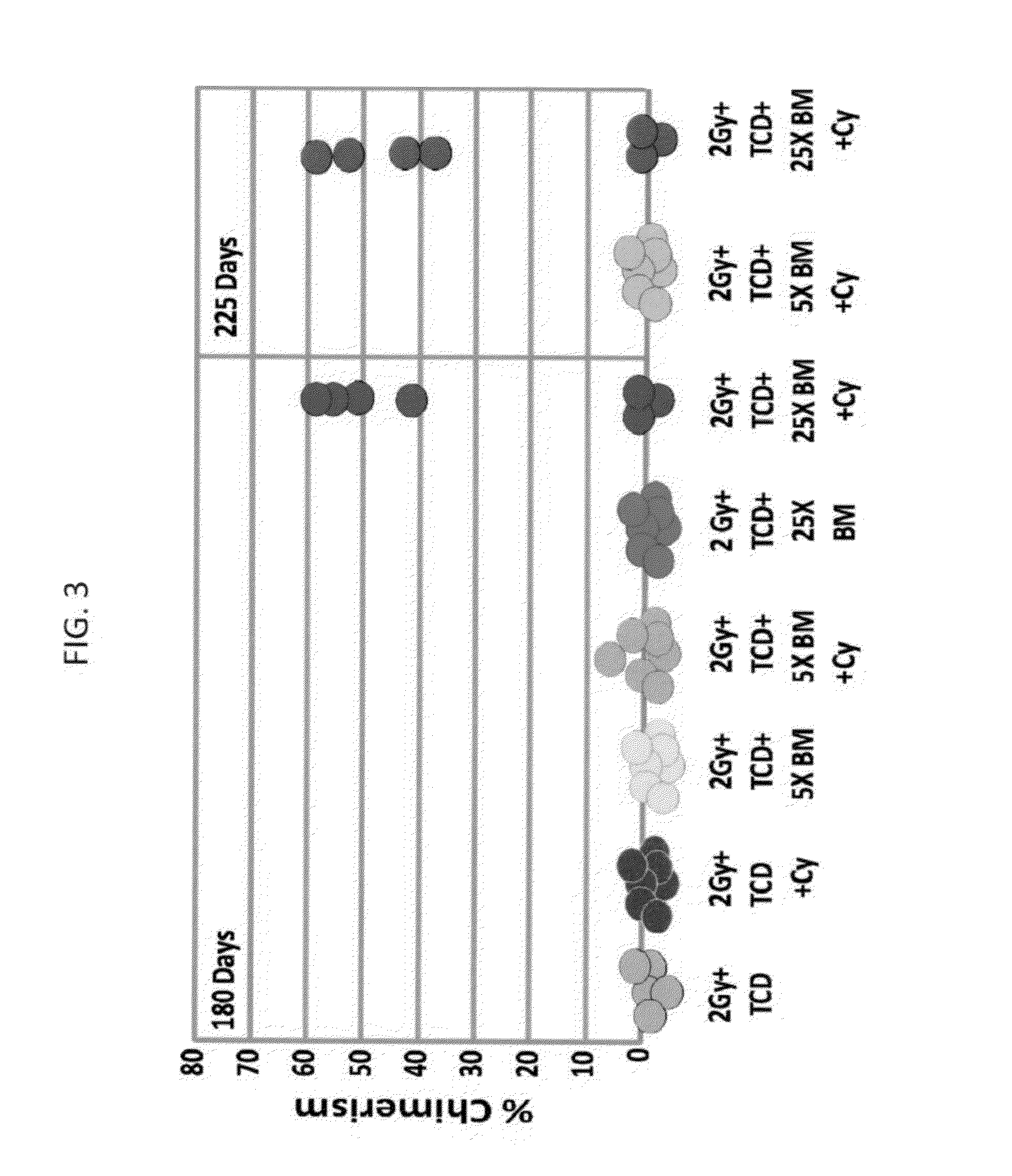
Combination therapy for a stable and long term engraftment using specific protocols for t/b cell depletion
A method of treating a subject in need of a non-syngeneic cell or tissue graft is disclosed. The methos comprising: (a) transplanting into a subject a dose of T cell depleted immature hematopoetic cells, wherein the T cell depleted immature hematopoetic cells comprise less than 5×105 CD3+ T cells per kilogram body weight of the subject, and wherein the dose comprises at least about 5×106 CD34+ cells per kilogram body weight of the subject, and wherein the T cell depleted immature hematopoetic cells are obtained by separating the T cells from the immature hematopoetic cells by magnetic cell sorting, and (b) administering to the subject a therapeutically effective amount of cyclophosphamide, wherein the therapeutically effective amount comprises 25-200 mg per body weight, thereby treating the subject.
Owner:YEDA RES & DEV CO LTD
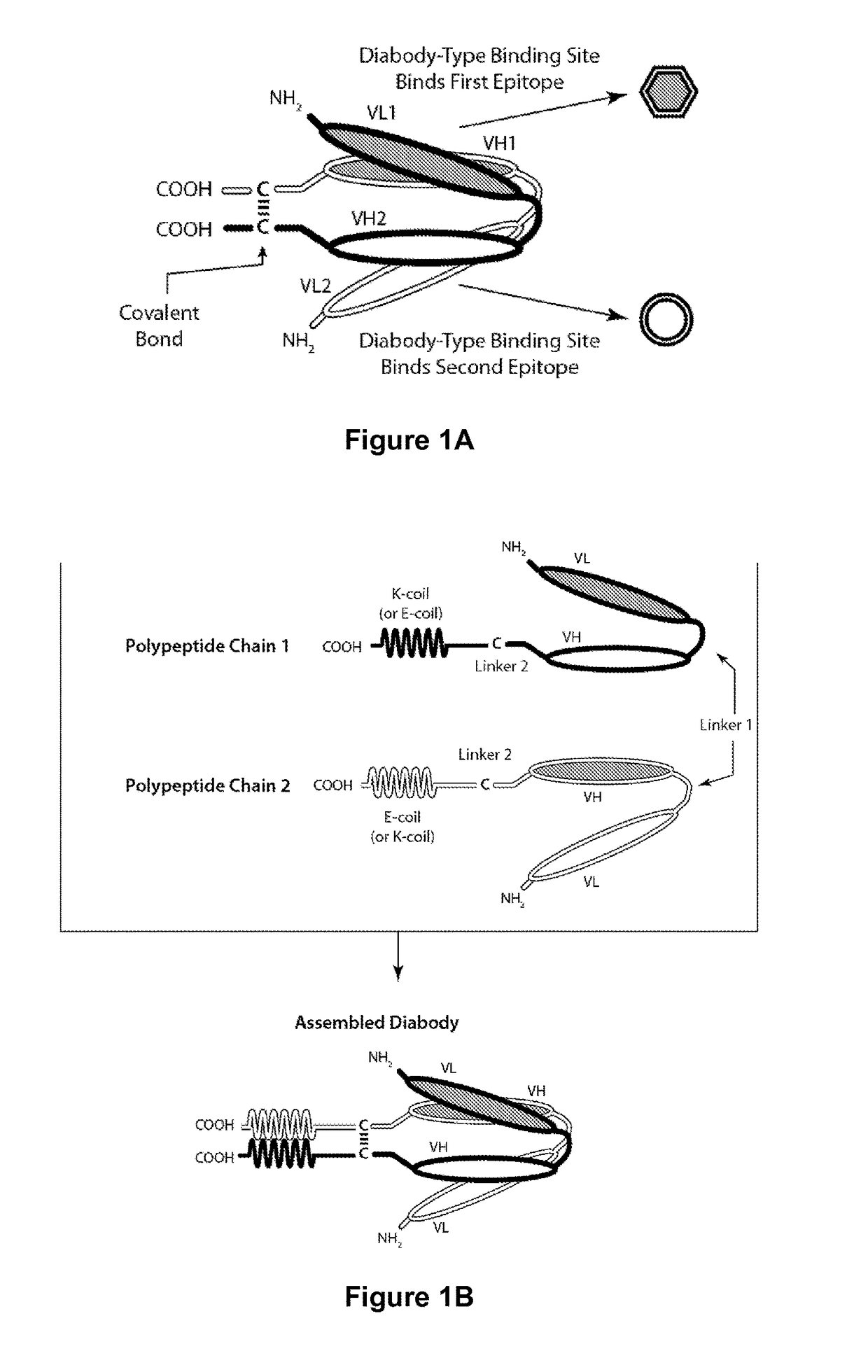
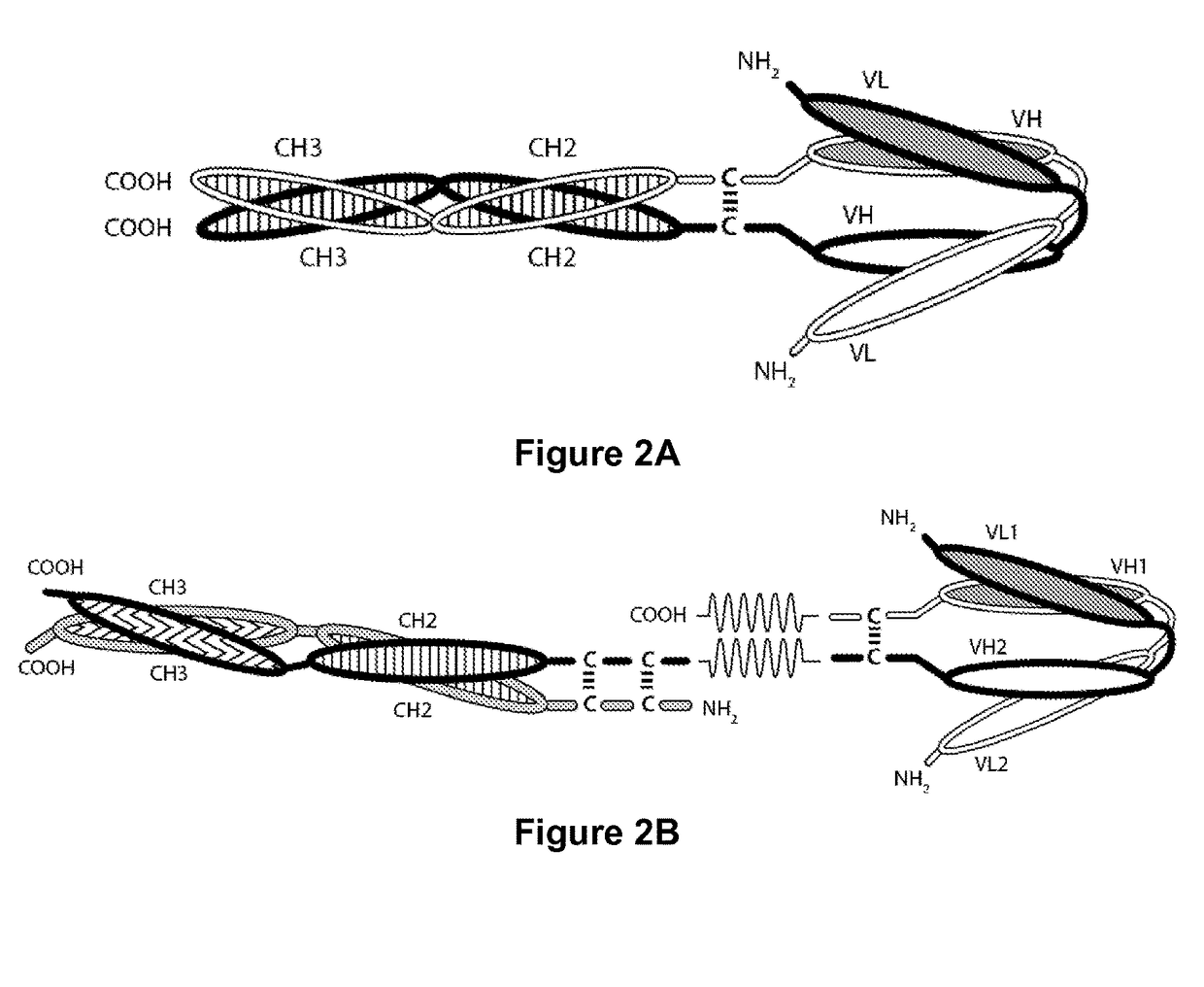
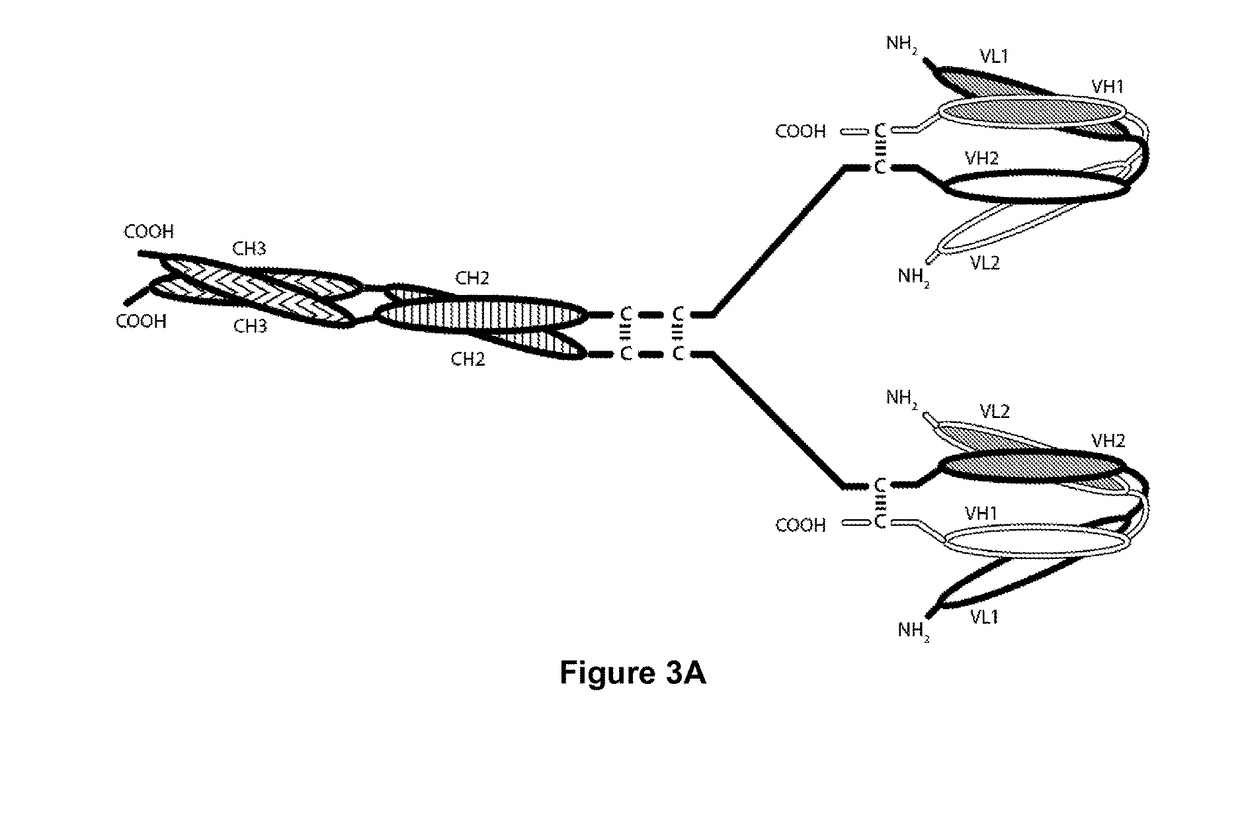
Tri-Specific Binding Molecules and Methods of Use Thereof
The present invention relates to Tri-Specific Binding Molecules, which are multichain polypeptide molecules that possess three Binding Domains and are thus capable of mediating coordinated binding to three epitopes. The Binding Domains may be selected such that the Tri-Specific Binding Molecules are capable of binding to any three different epitopes. Such epitopes may be epitopes of the same antigen or epitopes of two or three different antigens. In a preferred embodiment, one of such epitopes will be capable of binding to CD3, the second of such epitopes will be capable of binding to CD8, and the third of such epitopes will be capable of binding to an epitope of a Disease-Associated Antigen. The invention also provides a novel ROR1-binding antibody, as well as derivatives thereof and uses for such compositions.
Owner:MACROGENICS INC
Recombinant lentivirus and application thereof
ActiveCN106749675ASignificant in vivo and in vitro amplificationSignificant tumor killing effectMammal material medical ingredientsImmunoglobulinsAbnormal tissue growthMicro environment
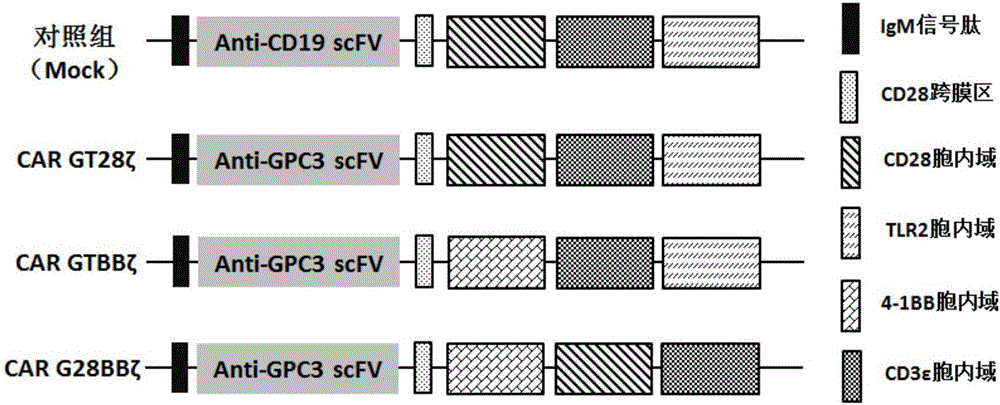
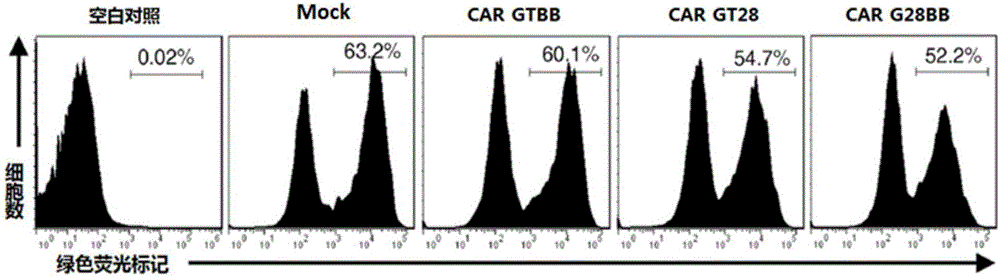
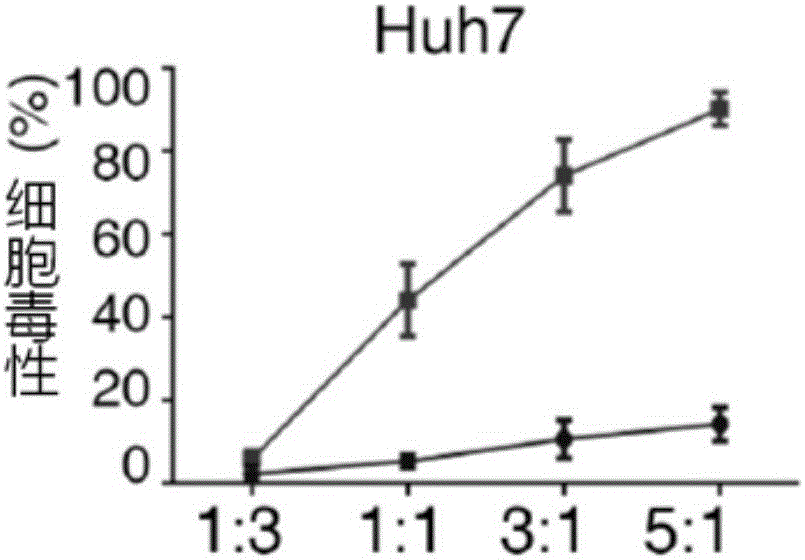
The invention relates to the field of tumor cellular immunotherapy, and in particular relates to a recombinant lentivirus and application thereof. The recombinant lentivirus comprises a chimeric antigen receptor, wherein the chimeric antigen receptor mainly comprises signal peptide, an antigen recognition domain, a transmembrane domain, an intracellular co-stimulation signal transduction domain and a CD3 zeta signal transduction domain which are serially connected; the intracellular co-stimulation signal transduction domain mainly comprises a human TLR2 (Toll Like Receptor 2) intracellular domain. A GPC3 CAT T (Glypican 3 CAT T) cell prepared from the recombinant lentivirus has an intense cell killing effect on liver cancer cells, a Th1 cell factor can be highly expressed, a tumor killing effect caused by non-CAR T (Chimeric Antigen Receptor T) cell can be stimulated to the maximum extent, escape and potential reoccurrence risk of GPC 3-tumor cells can be effectively prevented, the tumor cells can be killed by T cells expressing the chimeric antigen receptor, normal tissue can be slightly damaged, a tumor immunosuppression micro environment can be broken through, and thus a relatively good treatment effect on solid tumor can be achieved.
Owner:SHENZHEN IN VIVO BIOMEDICINE TECH LTD
Engineering and Delivery of Therapeutic Compositions of Freshly Isolated Cells
ActiveUS20090257991A1Improve biological activitySimple procedureGenetic material ingredientsFermentationNatural Killer Cell Inhibitory ReceptorsCD8
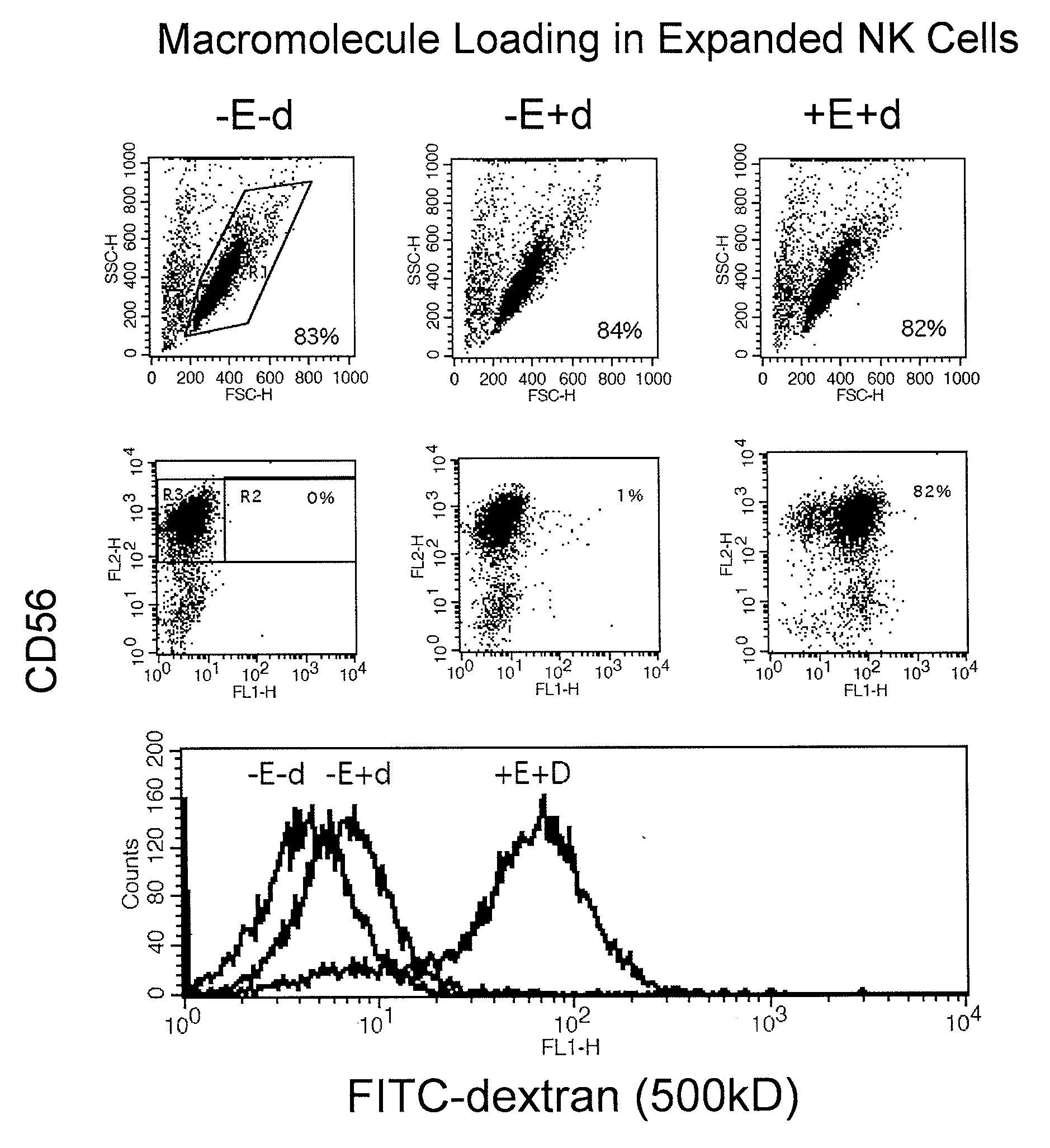
The present invention relates to the transient modification of cells. In particular embodiments, the cells are immune systems, such as PBMC, PBL, T (CD3+ and / or CD8+) and Natural Killer (NK) cells. The modified cells provide a population of cells that express a genetically engineered chimeric receptor which can be administered to a patient therapeutically. The present invention further relates to methods that deliver mRNA coding for the chimeric receptor to unstimulated resting PBMC, PBL, T (CD3+ and / or CD8+) and NK cells and which delivers the mRNA efficiently to the transfected cells and promotes significant target cell killing.
Owner:MAXCYTE
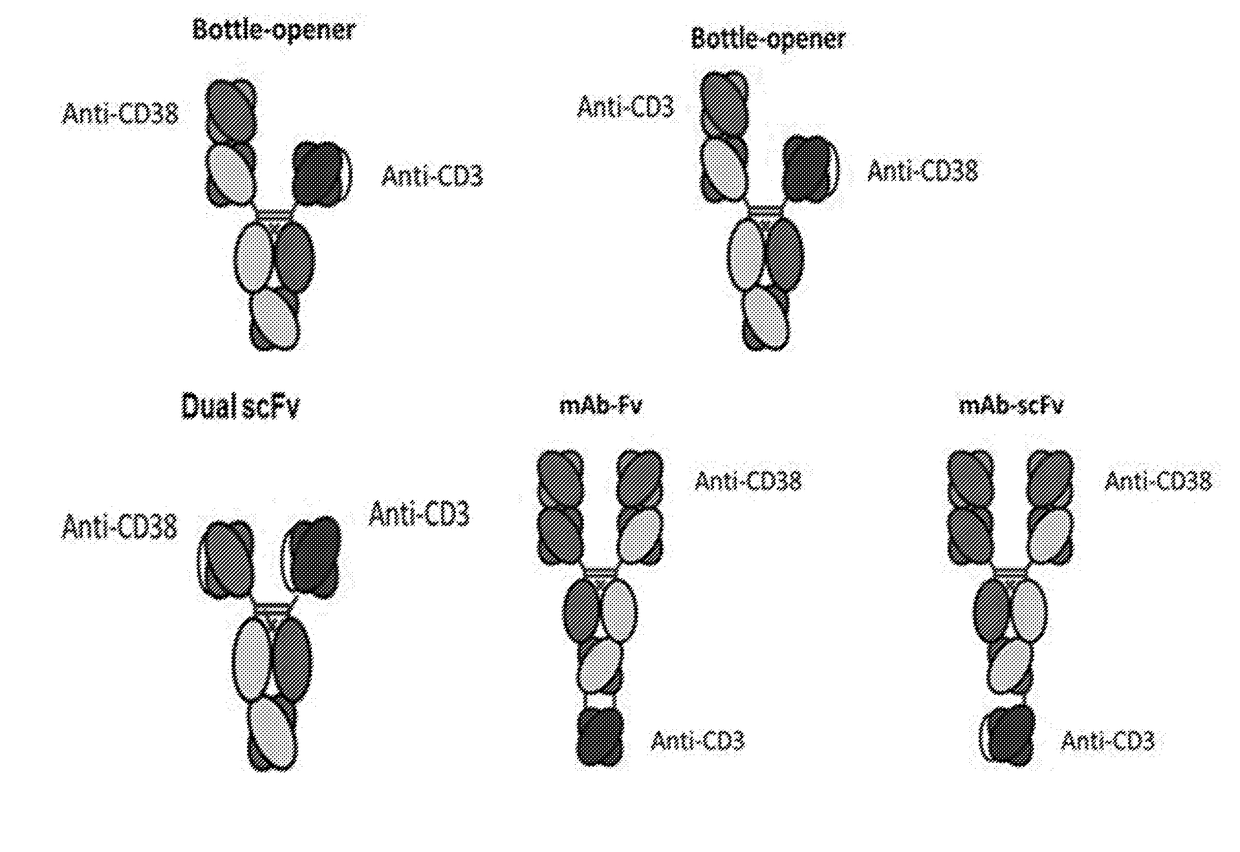
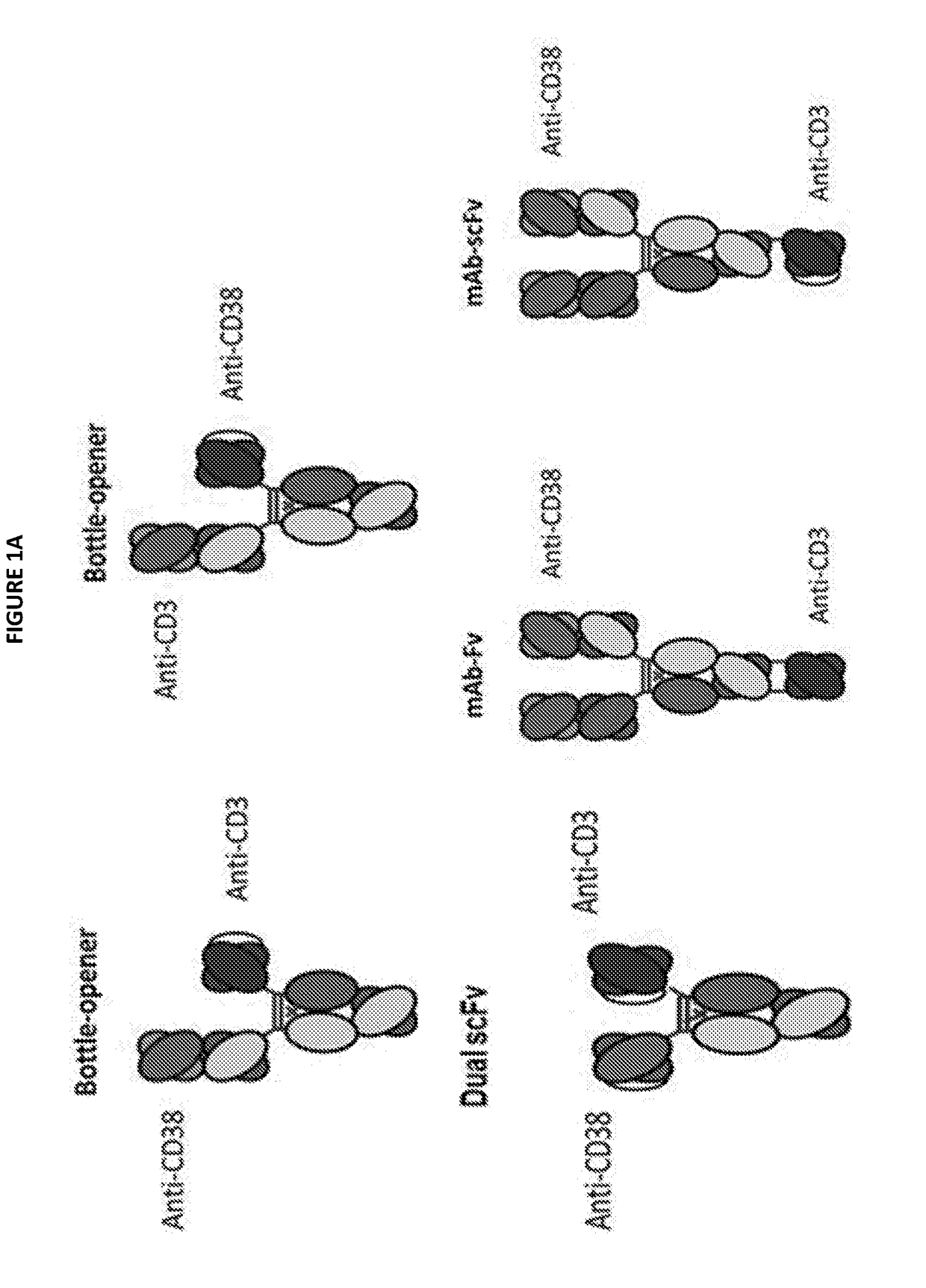
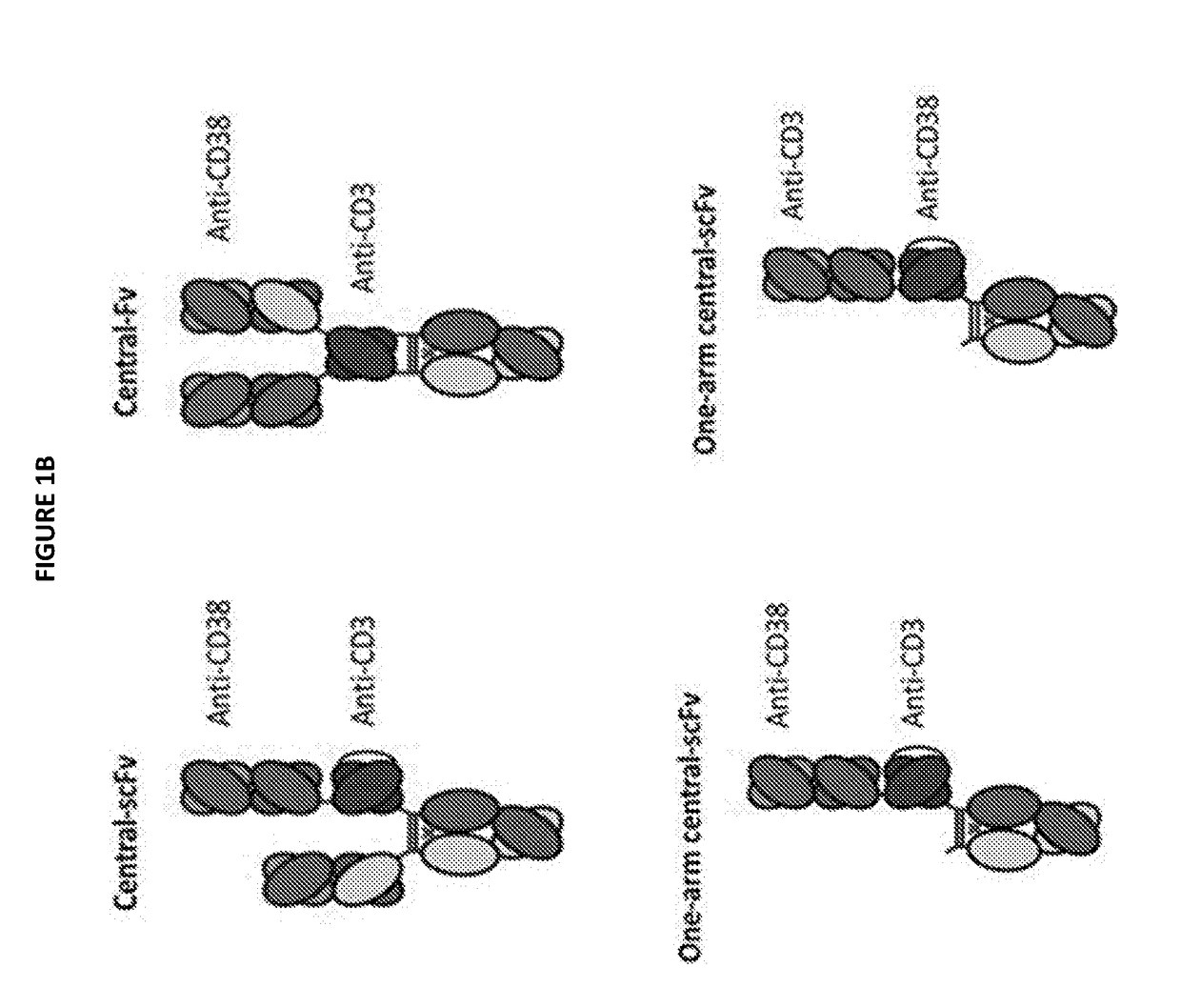
Heterodimeric antibodies that bind cd3 and cd38
InactiveUS20180305465A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyCD38
Owner:XENCOR
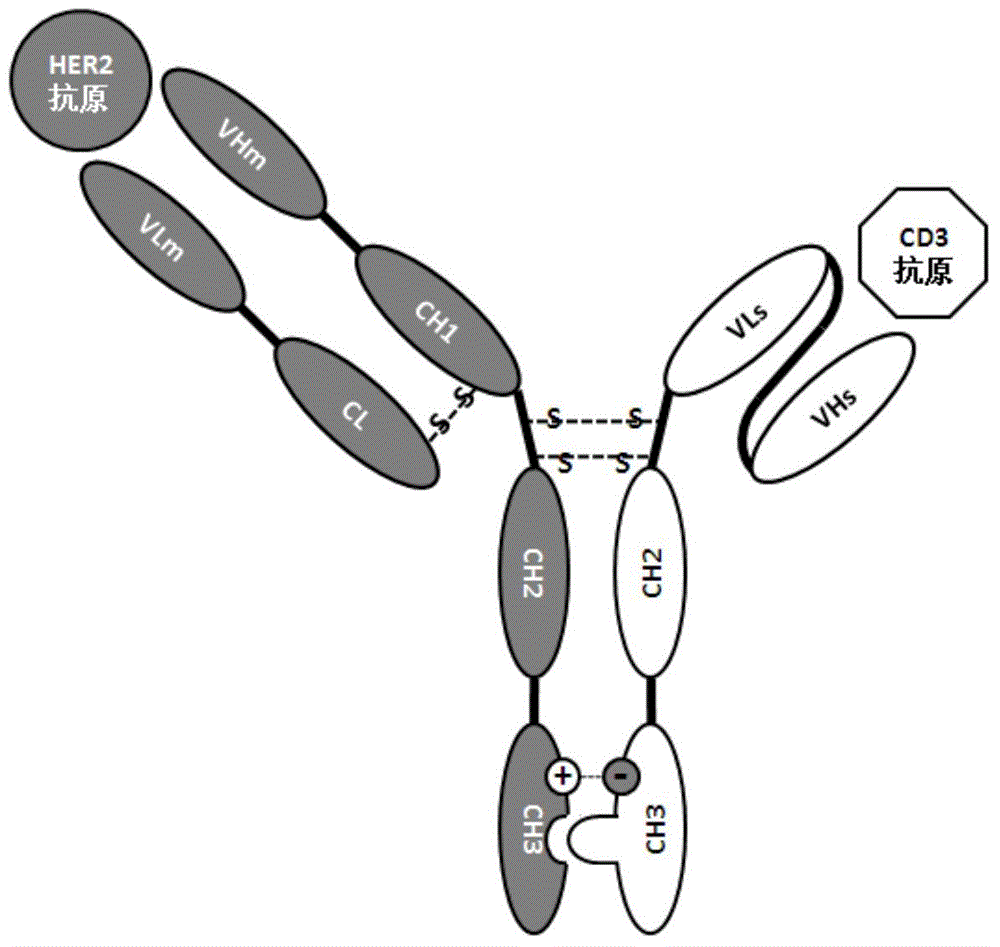
Construction and application of bispecific antibody HER2*CD3
ActiveCN104558192AIncrease lethalityIncreased immunotherapyHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenSingle-chain variable fragment
The invention provides a bispecific antibody, and particularly relates to construction and application of a bispecific antibody HER2*CD3. The bispecific antibody comprises a single-chain unit and a monovalent unit, wherein the single-chain unit has a specific binding capability against the surface antigen CD3 of an immune cell, and the monovalent unit has a specific binding capability against the surface antigen HER2 of a tumor cell; and the single-chain unit comprises a single-chain variable fragment (ScFv) which is fused with an Fc fragment, and the monovalent unit comprises light chain and heavy chain pairs. The invention further provides a method for preparing the bispecific antibody and the medicinal application of the antibody.
Owner:WUHAN YZY BIOPHARMA CO LTD
Bispecific antibodies against HER2 and CD3
Bispecific antibodies which comprise one antigen-binding region binding to an epitope of human epidermal growth factor receptor 2 (HER2) and one antigen-binding region binding to human CD3, and related antibody-based compositions and molecules, are disclosed. Pharmaceutical compositions comprising the antibodies and methods for preparing and using the antibodies are also disclosed.
Owner:健玛保
In-vitro culture method for T lymphocytes
ActiveCN102433303AKeep aliveExtended shelf lifeBlood/immune system cellsAntiendomysial antibodiesWhite blood cell
The invention discloses an in-vitro culture method for T lymphocytes, and radically solves the problems of high cost, inconvenience of use and unfavorable medical research and clinical application existing in the conventional in-vitro culture method for the T lymphocytes. The method comprises the following steps of: directionally sorting the T lymphocytes, inducing, differentiating, culturing, amplifying and the like. A special cell factor combination is used for performing induction differentiation on the T lymphocytes; three factors are a CD3 monoclonal antibody, gamma-interferon and interleukin-1 alpha respectively; the optimal concentration ratio of the three factors is 10:1:1; the total final concentration of the three factors in a culture medium is 1,200ng / ml; and the experiment shows that the combination is an extremely effective way of inducing the T lymphocytes to differentiate.
Owner:LIAONING MEDI BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com