Preparation method and application of PD-1/CTLA-4 (programmed death-1/cytotoxic T lymphocyte antigen-4) bispecific antibody
A CTLA-4, bispecific antibody technology, applied in the field of life sciences, can solve the problem that there is no efficient preparation method for bispecific antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The invention provides a method for preparing a PD-1 / CTLA-4 bispecific antibody gene fragment, comprising the following steps:
[0044] Step 1, using the anti-PD-1dsFv gene as a template to amplify the gene fragment PD-1V H -GGGGS, the sequences of upstream and downstream amplification primers are shown in SEQNO.1 and SEQNO.2;
[0045] Step 2, using the anti-CTLA-4dsFv gene as a template to amplify the gene fragment GGGGS-CTLA-4V L , the sequences of upstream and downstream amplification primers are shown in SEQNO.3 and SEQNO.4;
[0046] Step 3, OverlapPCR splicing step 1 and step 2 gene fragment PD-1V H -GGGGS and GGGGS-CTLA-4V L , construct the gene fragment PD-1V H -GGGGS-CTLA-4V L , the amplification primer sequence is shown in SEQNO.1 and SEQNO.5;
[0047] Step 4, using the anti-CTLA-4dsFv gene as a template to amplify the gene fragment CTLA-4V H -GGGGS, the sequences of upstream and downstream amplification primers are shown in SEQNO.6 and SEQNO.2;
[0048...
Embodiment 1
[0052] Example 1 Preparation of PD-1 / CTLA-4 bispecific antibody gene fragment
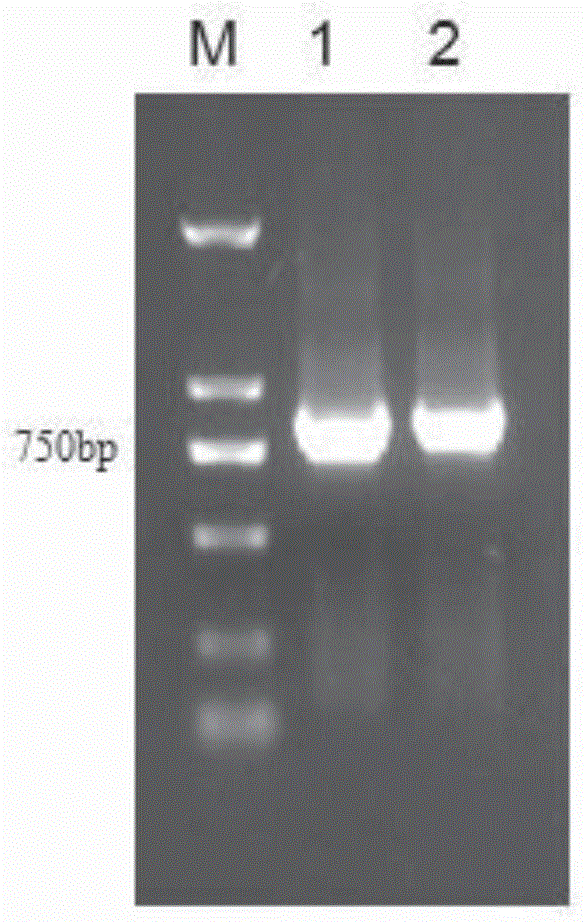
[0053] Using anti-PD-1 and CTLA-4DsFv genes as templates, construct PD-1V by PCR and OverlapPCR respectively H -GGGGS-CTLA-4V L , CTLA-4V H -GGGGS-PD-1V L Gene fragments, and then construct PD-1V through furin-GSGS-2A linker peptide H -GGGGS-CTLA-4V L -furin-GSGS-2A-CTLA-4V H -GGGGS-PD-1V L (if attached figure 1 ).
[0054] Table 1 PCR primer sequence
[0055] Tab.1 Primersequences
[0056]
[0057]
[0058] Step 1, gene fragment PD-1V H -Amplification of GGGGS
[0059] Using the DsFv gene as a template, use primer 1 and primer 2 to amplify the gene fragment PD-1V H -GGGGS, the amplification system is as follows:
[0060] Table 2 PCR reaction system
[0061] Table.2 PCR reaction system
[0062]
[0063] The PCR amplification program was: pre-denaturation at 98°C for 30s, denaturation at 98°C for 5s, extension at 72°C for 30s, and after 30 cycles, extension at 72°C for 5min. ...
Embodiment example 2
[0109] Example 2 Construction of PD-1 / CTLA-4 bispecific antibody lentiviral expression vector and packaging of recombinant lentivirus
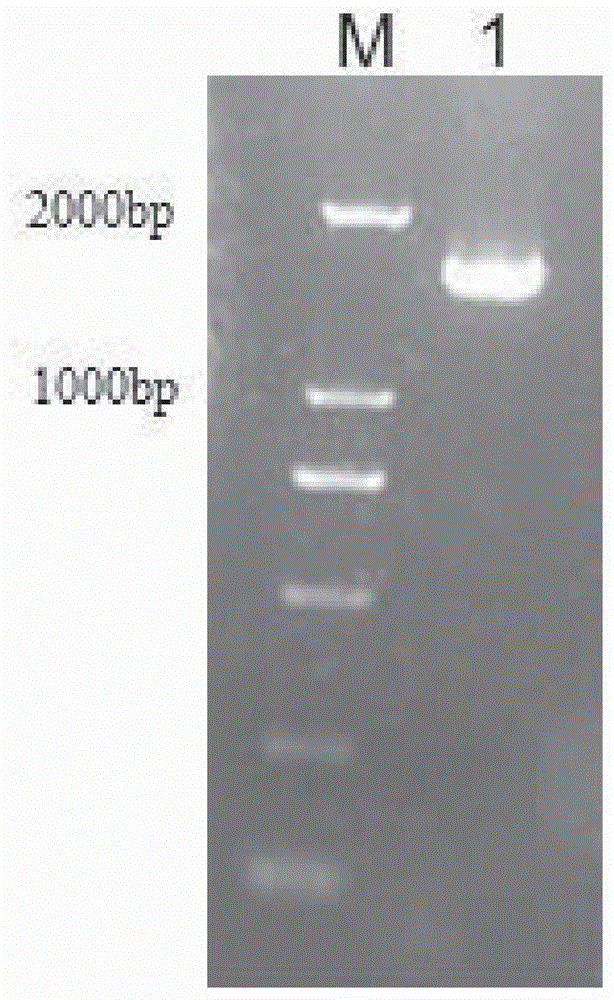
[0110] Use EcoRI and NotI respectively to obtain PD-1V in embodiment one H -GGGGS-CTLA-4V L -furin-GSGS-2A-CTLA-4V H -GGGGS-PD-1V L And the lentiviral expression vector was subjected to double digestion (Table 11, 12), digested at 37° C. for 8 hours, and the digested products were separated by 1% agarose gel electrophoresis and then recovered from the gel.
[0111] Table 11 enzyme digestion system
[0112] Table.11digestionsystem
[0113]
[0114] Table 12 enzyme digestion system
[0115] Table.12 digestion system
[0116]
[0117] Ligate the recovered gene fragments with the digested vector (Table 13), ligate at 16°C for 8 hours, take 5 μl of the ligated product and transform into 50 μL Escherichia coli DH5α, plate, pick a single colony and extract the plasmid, and finally use EcoRI and NotI to The plasmid was subjected to double...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com