Anti-PD1 monoclonal antibody, pharmaceutical composition and uses thereof
A monoclonal antibody, antibody technology, applied in the field of tumor treatment and molecular immunology, can solve problems such as liver damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
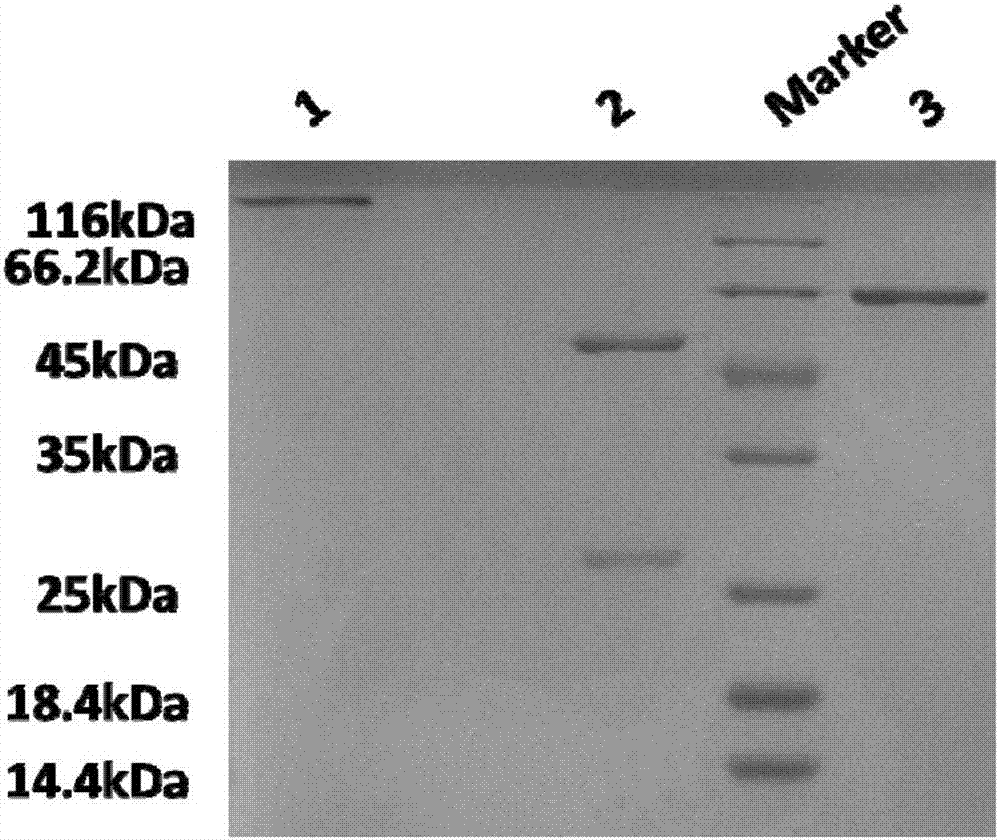
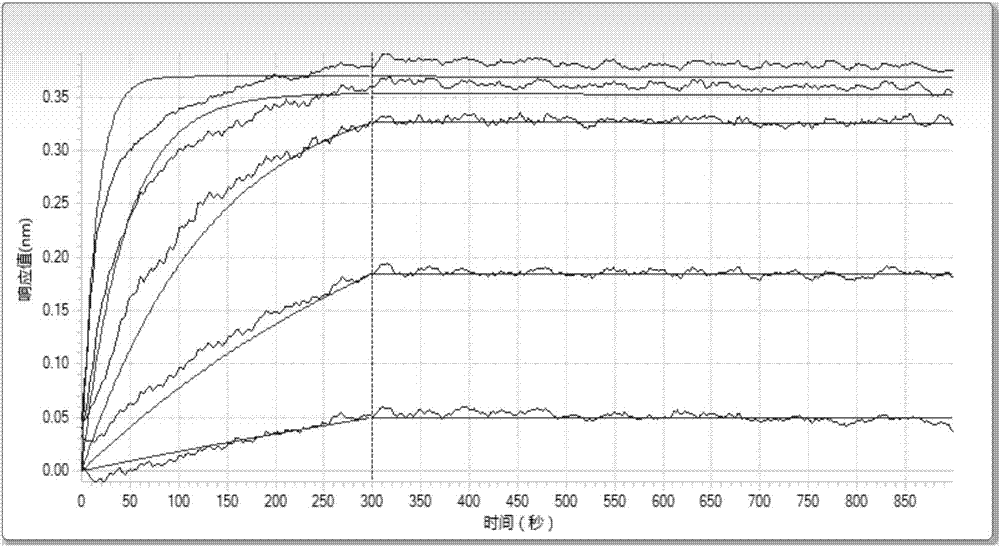
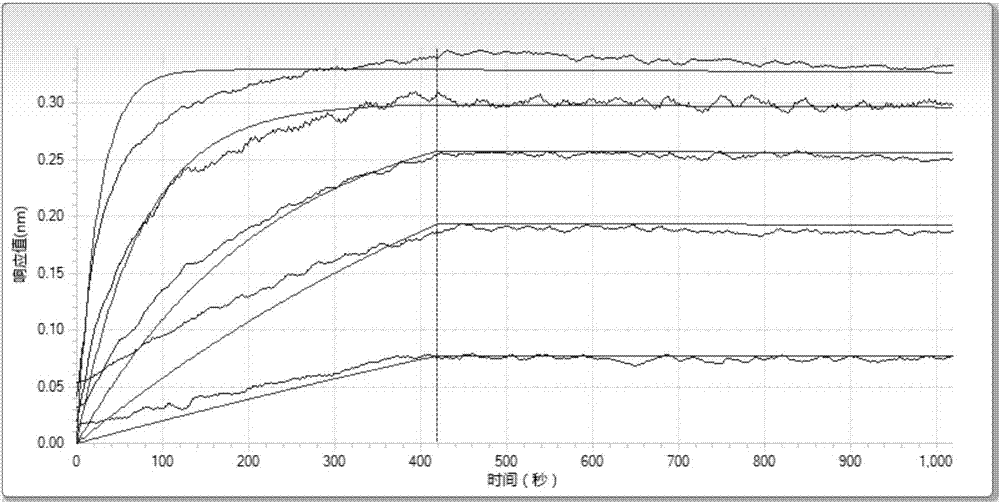
Image
Examples
Embodiment 1
[0116] Example 1: Obtaining of hybridoma cell line LT003 and preparation of monoclonal antibody 14C12
[0117] 1. Establishment of hybridoma cell line LT003
[0118] Using PD1-mFc (PD1: Programmed cell death protein 1, NCBI GenBank ID: NP_005009.2) fusion protein as an antigen, the splenocytes and mouse myeloma cells from immunized BALB / C mice (purchased from Guangdong Medical Experimental Animal Center) were collected. Cells are fused into hybridoma cells, referring to currently established methods (for example, Stewart, S.J., "Monoclonal Antibody Production", in Basic Methods in Antibody Production and Characterization, Eds.G.C.Howard and D.R.Bethell, Boca Raton: CRC Press, 2000) .
[0119] Using PD1-mFc as an antigen to coat the microtiter plate, performing indirect ELISA screening, and obtaining hybridoma cells secreting new antibodies specifically binding to PD1.
[0120] A hybridoma cell line that can secrete a monoclonal antibody that competes with the ligand PDL1-h...
Embodiment 2
[0124] Example 2: Obtaining the Light Chain and Heavy Chain Sequences of Monoclonal Antibody 14C12
[0125] The mRNA was extracted from the hybridoma cell line LT003 prepared in Example 1 according to the method of the Bacteria Total RNA Extraction Kit (Tiangen, Cat. No. DP430) for cultured cells.
[0126] According to Invitrogen III First-Strand Synthesis System for RT-PCR Kit Instructions Synthesize cDNA and perform PCR amplification.
[0127] The PCR amplification product was directly cloned by TA, and the specific operation was performed referring to the instructions of the pEASY-T1 Cloning Kit (TransgenCT101) kit.
[0128] The products of TA clones were directly sequenced, and the sequencing results were as follows:
[0129] DNA sequencing results of heavy chain variable region: (354bp)
[0130] GAGGTCAAACTGGTGGAGAGCGGCGGCGGGCTGGTGAAGCCCGGCGGGTCACTGAAACTGAGCTGCGCCGCTTCCGGCTTCGCCTTTAGCTCCTACGACATGTCATGGGTGAGGCAGACCCCTGAGAAGCGCCTGGAATGGGTCGCTACTATCAGCGGAGGCGGGCGATACA...
Embodiment 3
[0142] Example 3: Design of heavy chain and light chain sequences of humanized antibody 14C12H1L1
[0143] 1. Design of light chain and heavy chain sequences of humanized antibody 14C12H1L1
[0144] According to the three-dimensional crystal structure of the PD1 protein (Shinohara T, et al., Structure and chromosomal localization of the human PD1 gene (PDCD1). Genomics1995,23(3):704-6) and the sequence of the antibody 14C12 obtained in Example 2, by The antibody model was simulated by computer, and mutations were designed according to the model to obtain the variable region sequence of antibody 14C12H1L1 (heavy chain constant region is Ig gamma-1 chain C region, ACCESSION: P01857, light chain constant region is Ig kappa chain C region, ACCESSION: P01834 ), the variable region sequence is as follows:
[0145] DNA sequence of heavy chain variable region: (354bp)
[0146] GAAGTGCAGCTGGTCGAGTCTGGGGGAGGGCTGGTGCAGCCCGGCGGGTCACTGCGACTGAGCTGCGCAGCTTCCGGATTCGCCTTTAGCTCCTACGACATGTCC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com