Anti-PD-L1 antibody as well as pharmaceutical composition and application of anti-PD-L1 antibody
A PD-L1, antibody technology, applied in the field of tumor therapy and molecular immunology, can solve the problem of tumor cell escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: PD-L1 full antibody expression
[0096] Synthesis of Mil85-6 heavy chain variable region gene and light chain variable region gene. The gene sequence is as follows:
[0097] Nucleic acid sequence encoding Mil85-6 heavy chain variable region
[0098] GAGGTGCAGCTGGTGGAGAGCGGCGGCGGCCTGGTGCAGCCAGGCGGCAGCCTGAGACTGAGCTGCGCCGCCAGCGGCTTCACCTTCGCTGATAGCTGGATCCACTGGGTGAGACAGGCCCCTGGCAAGGGCCTGGAGTGGGTGGCCTGGATCAGCCCATTTGGCGGCTCTAATTACTACGCCGACAGCGTGAAGGGCAGATTCACCATCAGCGCCGACACCAGCAAGAACACCGCCTACCTGCAGATGAACAGCCTGAGAGCCGAGGACACCGCCGTGTACTACTGCGCCAGAAGACACTGGCCAGGCGGCTTCGACTACTGGGGCCAGGGCACCCTGGTGACCGTGAGCAGC (SEQ ID NO: 9)
[0099] Nucleic acid sequence encoding Mil85-6 light chain variable region
[0100] GATATCCAGATGACCCAGAGCCCGAGCAGCCTGAGCGCGAGCGTGGGTGATCGCGTGACCATTACCTGCCGCGCGAGCCAGAGTATCGGTACCGCCCTGAATTGGTATCAGCAGAAACCGGGTAAAGCGCCGAAACTGTTAATTTATACGGCCAGCAGCCTGCAGTCTGGCGTGCCGTCGCGTTTTAGCGGCTCGGGTTCGGGCACCGATTTTACCCTGACCATCTCGAGCTTGCAGCCGGAGGACTTCGCCACCTACTATTGCCAGCAAGACAAC...
Embodiment 2
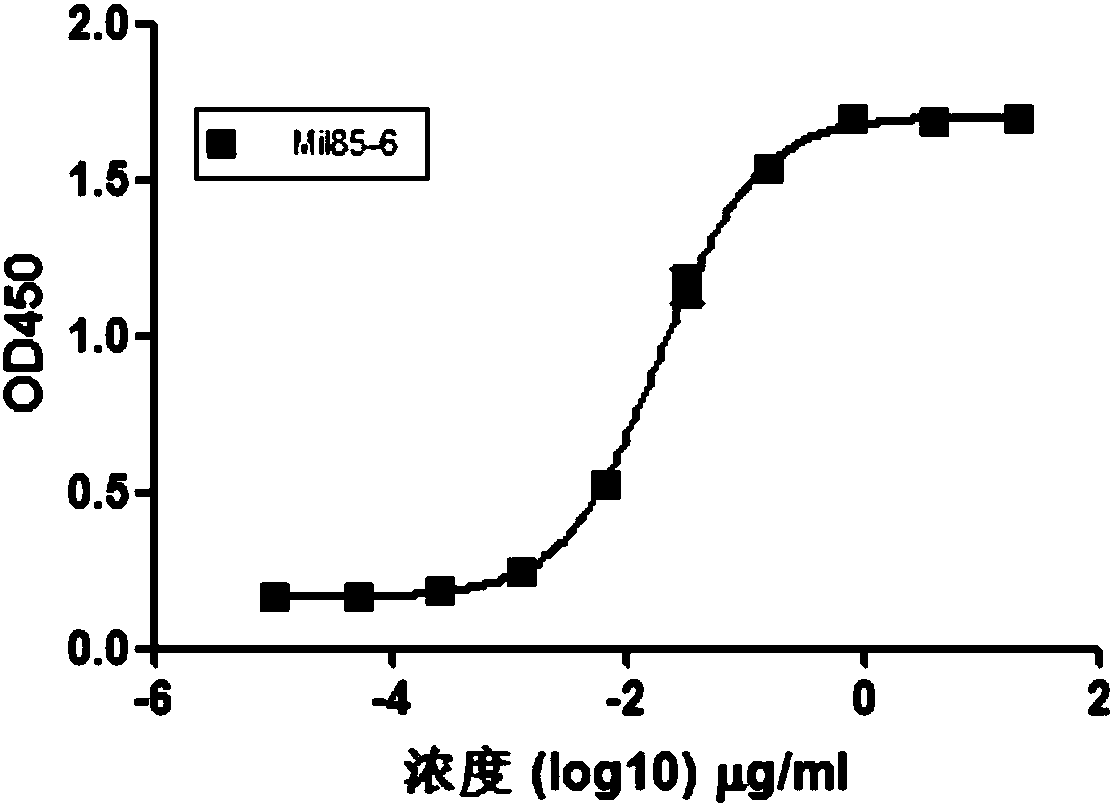
[0102] Example 2: Binding of whole antibody to PD-L1
[0103] Coat 5μg / ml PD-L1-Fc with PBS in a 96-well plate, incubate overnight at 4°C, discard the coating solution the next day, and wash three times with PBST, add 200μl blocking solution to each well, incubate at room temperature for 1h, PBST Wash three times, adjust the highest concentration of MIL85-6 antibody to 20μg / ml, and perform 5-fold dilution, make ten concentration gradients, add 100μl to each well, incubate for 1h at room temperature, wash three times with PBST, and add anti-Fab- HRP, 100μl per well, incubate at room temperature for 1h, wash each well four times, add TMB color developing solution, 100μl / well, develop color at room temperature, use 10% H 2 SO 4 Stop color development, 50μl / well, 450nm reading. Wherein, the used PD-L1-Fc can be prepared by a method known to those skilled in the art, for example, refer to the following steps:
[0104] The cDNA sequence of the extracellular region of the antigen PD-L1 ...
Embodiment 3
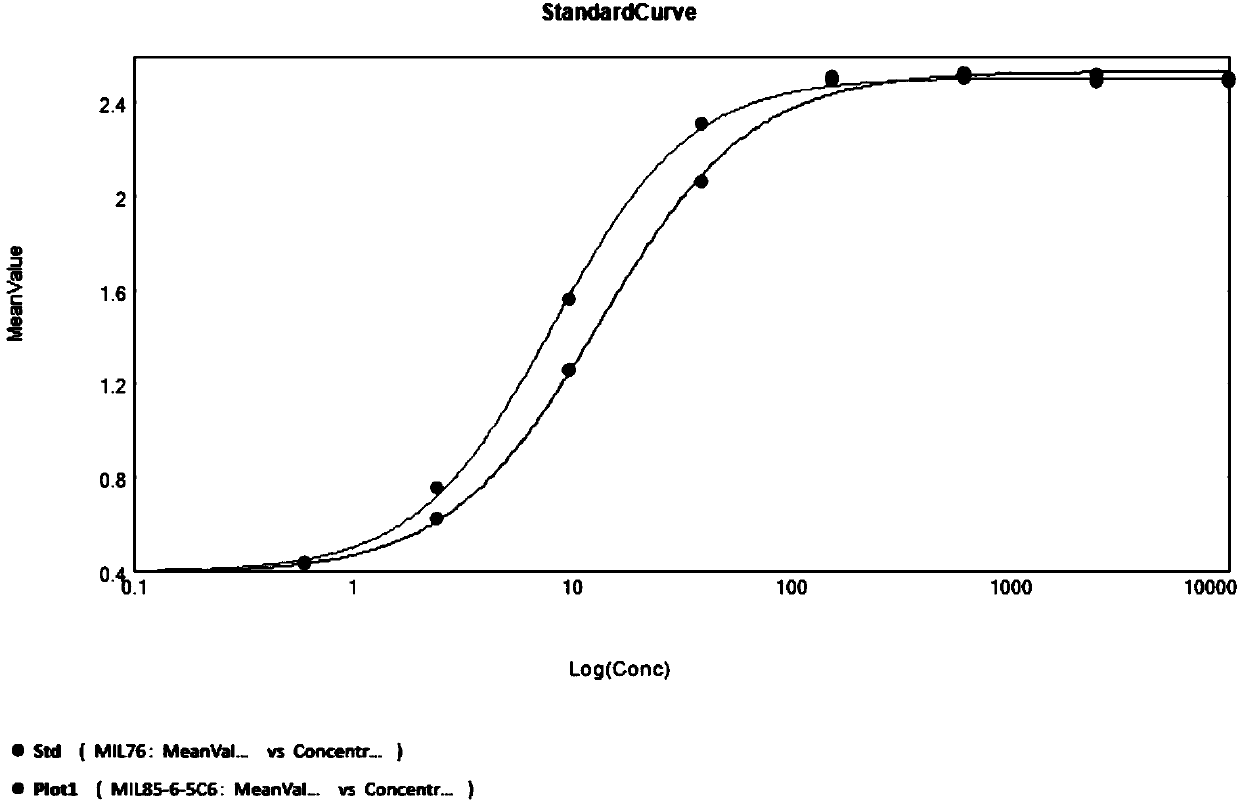
[0107] Example 3: Comparison of binding activity of whole antibodies
[0108] With Mil76 as a control (the sequence is referenced to Atezolizumab, and its expression and purification are obtained according to the expression and purification method of the MIL85-6 antibody), the affinity of the anti-PD-L1 antibody Mil85-6 obtained in the present invention is compared.
[0109] Coat 1μg / ml PD-L1-Fc with PBS in a 96-well plate, incubate overnight at 4°C, discard the coating solution the next day, and wash three times with PBST, add 200μl blocking solution to each well and incubate for 1h at room temperature. Wash three times with PBST, adjust the highest antibody concentration to 10μg / ml, and carry out 4-fold dilution, make 8 concentration gradients, add 100μl per well, incubate for 1h at room temperature, wash three times with PBST and add anti-Fab-HRP, 100μl per well, incubate for 1h at room temperature, wash four times, add TMB color developing solution, 100μl / well, develop color a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com