Anti-PD-1-anti-VEGFA bispecific antibodies, pharmaceutical compositions and uses thereof
A bispecific antibody, PD-1 technology, applied in the field of tumor therapy and immunology biology, can solve the problems of poor prognosis, unrealizable metastasis, and high late metastasis rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
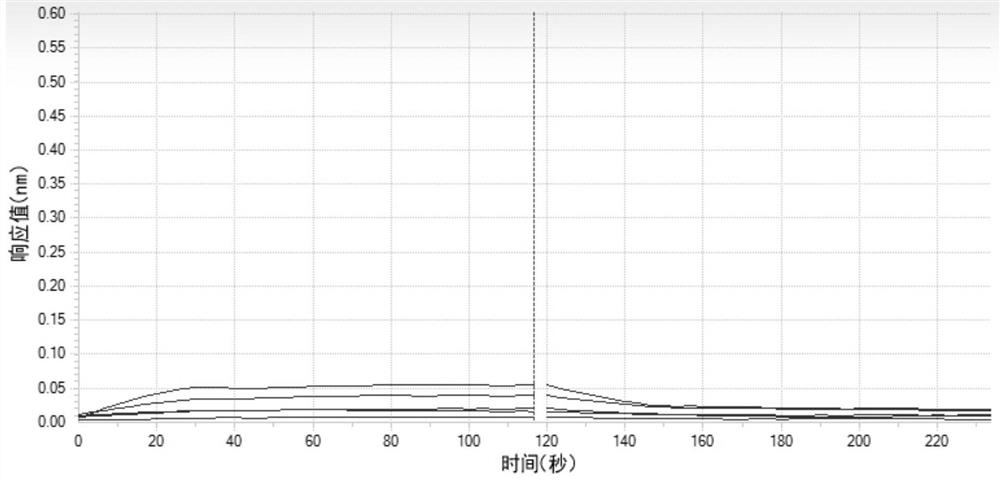
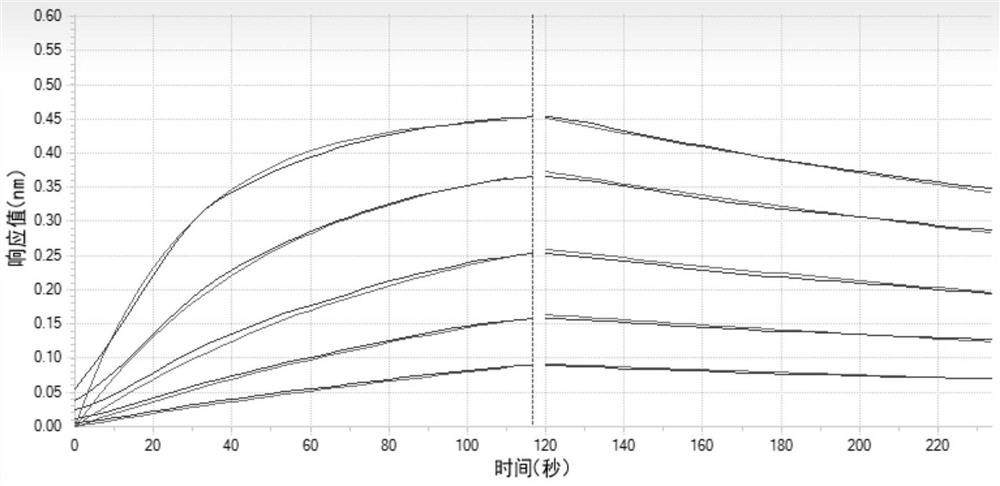
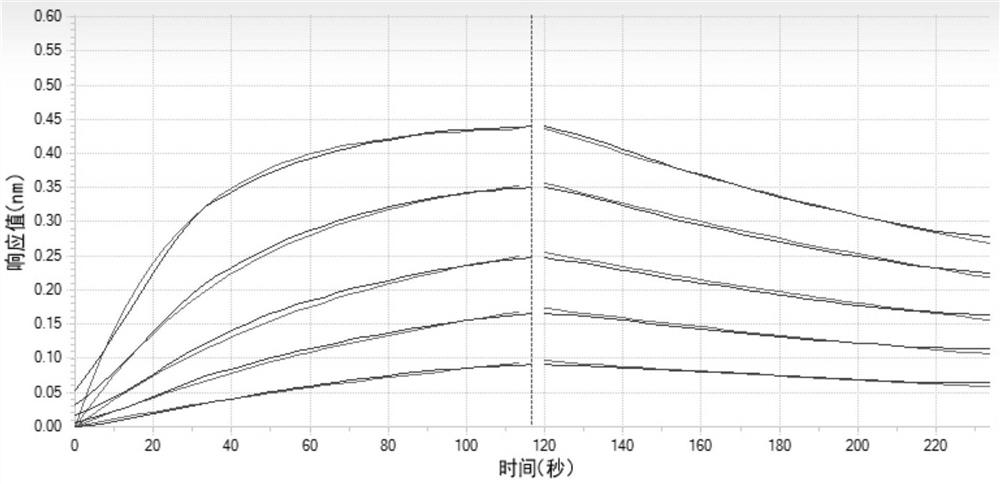
Image
Examples
preparation example 1
[0327] Preparation Example 1: Preparation of Anti-VEGFA Antibody Bevacizumab
[0328] For the amino acid sequences of the heavy chain variable region and the light chain variable region of the marketed anti-VEGFA monoclonal antibody Avastin (Bevacizumab), refer to Chinese patent publication CN1259962A. The nucleic acid sequences encoding the variable region of the heavy chain and the variable region of the light chain were synthesized by GenScript.
[0329] Amino acid sequence of Bevacizumab heavy chain variable region (Bevacizumab-Hv): (123aa)
[0330] EVQLVESGGGLVQPGGSLRLSCAASGYTFTNYGMNWVRQAPGKGLEWVGWINTYTGEPTYAADFKRRFTFSLDTSKSTAYLQMNSLRAEDTAVYYCAKYPHYYGSSHWYFDVWGQGTLVTVSS (SEQ ID NO: 1)
[0331] Nucleic acid sequence encoding Bevacizumab heavy chain variable region: (369bp)
[0332] GAGGTGCAGCTGGTCGAGTCCGGGGGGGGGCTGGTGCAGCCAGGCGGGTCTCTGAGGCTGAGTTGCGCCGCTTCAGGGTACACCTTCACAAACTATGGAATGAATTGGGTGCGCCAGGCACCAGGAAAGGGACTGGAGTGGGTCGGCTGGATCAACACTTACACCGGGGAACCTACCTATGCAGCCGACTTT...
preparation example 2
[0340] Preparation Example 2: Anti-PD-1 antibody 14C12, its humanized antibody 14C12H1L1 and mutant 14C12H1L1(M) sequence design
[0341] The amino acid sequences of the heavy and light chains of the anti-PD-1 antibody 14C12 and its humanized antibody 14C12H1L1, as well as the coding nucleic acid sequences are the same as those of 14C12 and 14C12H1L1 in Chinese Patent Publication CN 106967172A.
[0342] (1) Heavy chain variable region sequence and light chain variable region sequence of 14C12
[0343] Amino acid sequence of the heavy chain variable region of 14C12: (118aa)
[0344] EVKLVESGGGLVKPGGSLKLSCAASGFAFSSYDMSWVRQTPEKRLEWVATISGGGRYTYYPDSVKGRFTISRDNARNNTLYLQMSSLRSEDTALYYCANRYGEAWFAYWGQGTLVTVSA (SEQ ID NO: 5)
[0345] Nucleic acid sequence encoding the heavy chain variable region of 14C12: (354bp)
[0346] GAGGTCAAACTGGTGGAGAGCGGCGGCGGGCTGGTGAAGCCCGGCGGGTCACTGAAACTGAGCTGCGCCGCTTCCGGCTTCGCCTTTAGCTCCTACGACATGTCATGGGTGAGGCAGACCCCTGAGAAGCGCCTGGAATGGGTCGCTACTATCAGCGGAGGC...
preparation example 3
[0374] Preparation Example 3: Sequence Design of Bispecific Antibody
[0375] 1. Sequence Design
[0376] The structural mode of the bispecific antibody in the present invention belongs to the Morrison mode (IgG-scFv), that is, the C-terminus of the two heavy chains of an IgG antibody is connected to the scFv fragment of another antibody, and the main components of the heavy chain and the light chain are The compositional design is shown in Table 1 below.
[0377] On the basis of the above-mentioned Bevacizumab, the VP101 antibody with the amino acid sequences of the heavy chain variable region and light chain variable region of the above-mentioned 14C12H1L1(M) as the ScFv fragment is called VP101(M). Compared with 14C12H1L1, 14C12H1L1(M) effectively optimizes the structure of the bispecific antibody and improves its effectiveness.
[0378] Table 1: Compositional design of the heavy and light chains of VP101(M) and VP101(G4M)
[0379]
[0380]
[0381] In Table 1 ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
| Affinity constant | aaaaa | aaaaa |
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com